Abstract
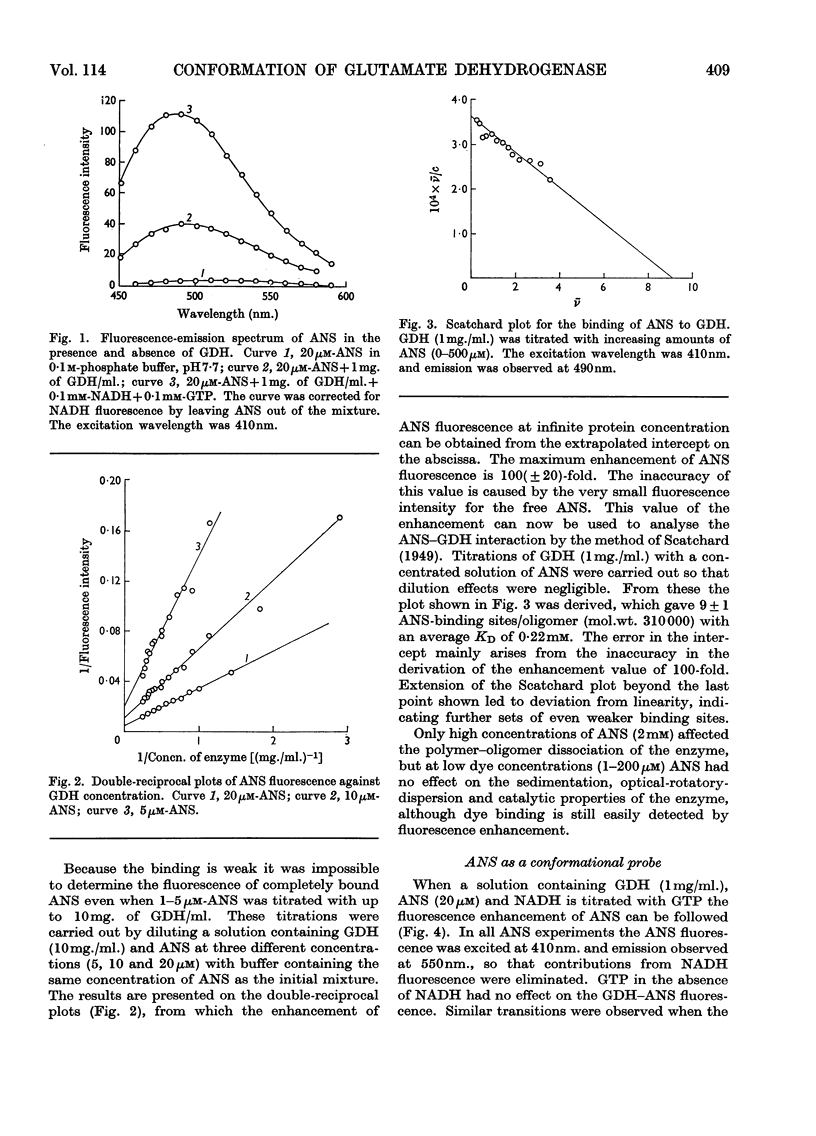
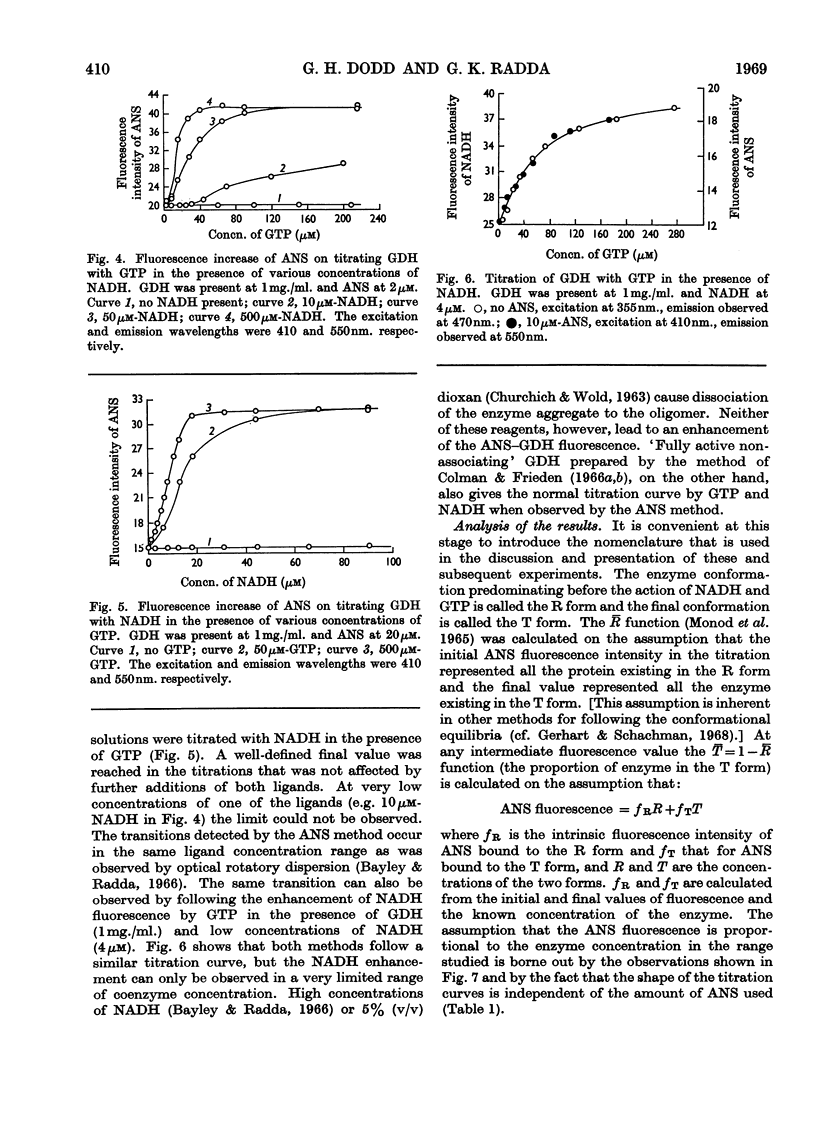
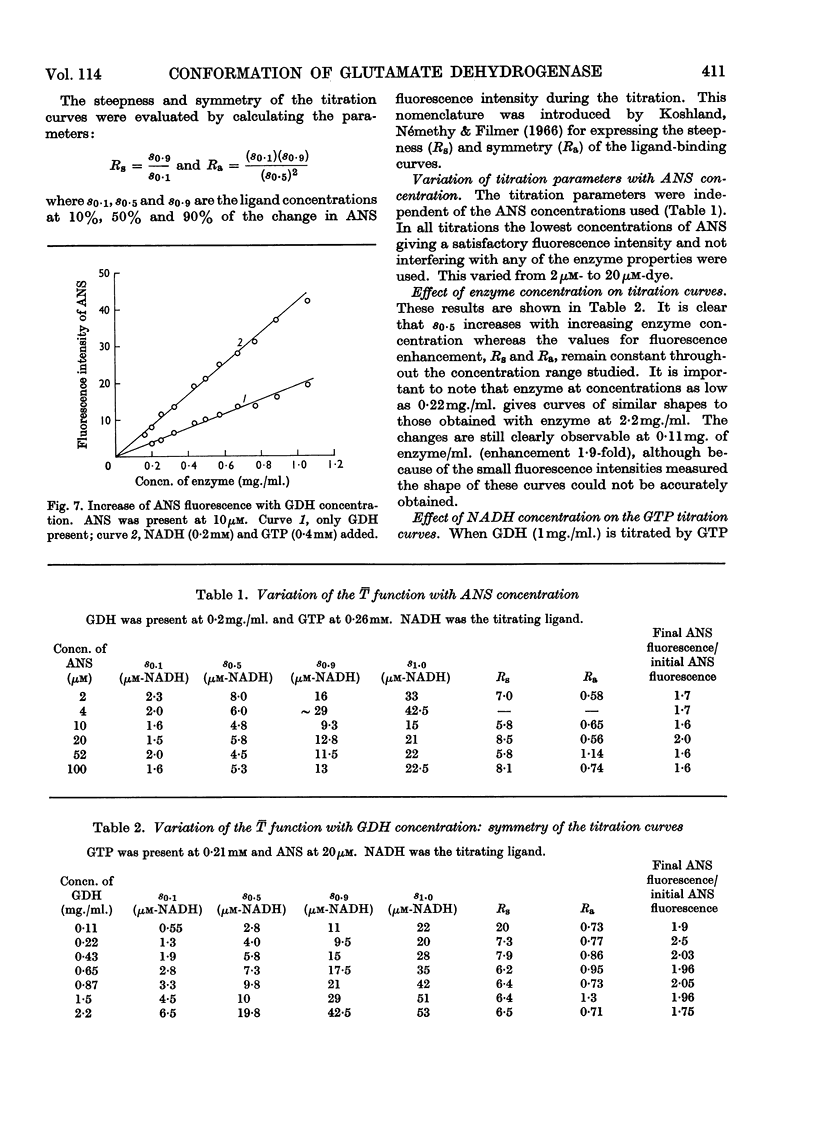
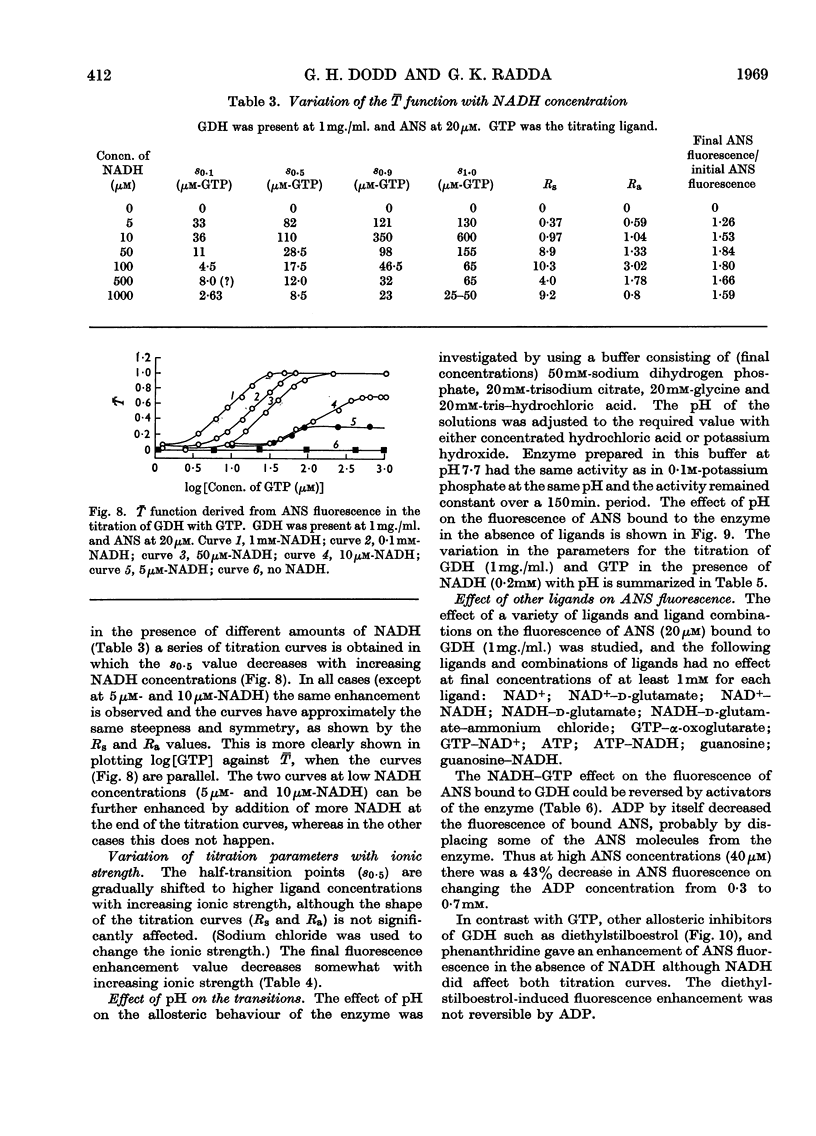
1. The interaction of 1-anilinonaphthalene-8-sulphonate with ox liver glutamate dehydrogenase was examined. 2. The fluorescence of the dye is enhanced 100-fold on binding. 3. A further enhancement is observed when NADH and GTP are added to the enzyme. 4. By using this property of the dye to measure conformational equilibria in the enzyme the effects of coenzyme, inhibitors, enzyme concentration, ionic strength and pH on the allosteric transitions were studied. 5. GTP and NADH interact with the enzyme in a heterotropic manner. 6. The rate of the structural transition brought about by GTP and NADH is biphasic with half-lives of 34 and 200msec. 7. The relation of these observations to regulatory mechanisms is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson B. M., Anderson C. D., Churchich J. E. Inhibition of glutamic dehydrogenase by pyridoxal 5'-phosphate. Biochemistry. 1966 Sep;5(9):2893–2900. doi: 10.1021/bi00873a017. [DOI] [PubMed] [Google Scholar]
- Bayley P. M., Radda G. K. Conformational changes and the regulation of glutamate-dehydrogenase activity. Biochem J. 1966 Jan;98(1):105–111. doi: 10.1042/bj0980105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHURCHICH J. E., WOLD F. THE EFFECT OF DIOXANE ON THE DISSOCIATION AND ACTIVITY OF GLUTAMIC DEHYDROGENASE. Biochemistry. 1963 Jul-Aug;2:781–786. doi: 10.1021/bi00904a027. [DOI] [PubMed] [Google Scholar]
- Changeux J. P., Gerhart J. C., Schachman H. K. Allosteric interactions in aspartate transcarbamylase. I. Binding of specific ligands to the native enzyme and its isolated subunits. Biochemistry. 1968 Feb;7(2):531–538. doi: 10.1021/bi00842a007. [DOI] [PubMed] [Google Scholar]
- Colman R. F., Frieden C. On the role of amino groups in the structure and function of glutamate dehydrogenase. I. Effect of acetylation on catalytic and regulatory properties. J Biol Chem. 1966 Aug 25;241(16):3652–3660. [PubMed] [Google Scholar]
- Colman R. F., Frieden C. On the role of amino groups in the structure and function of glutamate dehydrogenase. II. Effect of acetylation on molecular properties. J Biol Chem. 1966 Aug 25;241(16):3661–3670. [PubMed] [Google Scholar]
- Corman L., Kaplan N. O. Kinetic studies of dogfish liver glutamate dehydrogenase with diphosphopyridine nucleotide and the effect of added salts. J Biol Chem. 1967 Jun 25;242(12):2840–2846. [PubMed] [Google Scholar]
- Di Prisco G. Desensitization of the allosteric sites of glutamate dehydrogenase by fluorodinitrobenzene. Biochem Biophys Res Commun. 1967 Jan 23;26(2):148–152. doi: 10.1016/0006-291x(67)90226-4. [DOI] [PubMed] [Google Scholar]
- Dodd G. H., Radda G. K. Interaction of glutamate dehydrogenase with fluorescent dyes. Biochem Biophys Res Commun. 1967 May 25;27(4):500–504. doi: 10.1016/s0006-291x(67)80014-7. [DOI] [PubMed] [Google Scholar]
- FISHER H. F., McGREGOR L. L., PGWER U. The nature of the alkaline dissociation of the glutamic dehydrogenase molecule. Biochem Biophys Res Commun. 1962 Aug 7;8:402–406. doi: 10.1016/0006-291x(62)90016-5. [DOI] [PubMed] [Google Scholar]
- FRIEDEN C. GLUTAMATE DEHYDROGENASE. V. THE RELATION OF ENZYME STRUCTURE TO THE CATALYTIC FUNCTION. J Biol Chem. 1963 Oct;238:3286–3299. [PubMed] [Google Scholar]
- Gibson Q. H., Milnes L. Apparatus for rapid and sensitive spectrophotometry. Biochem J. 1964 Apr;91(1):161–171. doi: 10.1042/bj0910161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber J. E., Koshland D. E., Jr Relation of protein subunit interactions to the molecular species observed during cooperative binding of ligands. Proc Natl Acad Sci U S A. 1967 Nov;58(5):2087–2093. doi: 10.1073/pnas.58.5.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirtley M. E., Koshland D. E., Jr Models for cooperative effects in proteins containing subunits. Effects of two interacting ligands. J Biol Chem. 1967 Sep 25;242(18):4192–4205. [PubMed] [Google Scholar]
- Koshland D. E., Jr, Némethy G., Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966 Jan;5(1):365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- Malcolm A. D., Radda G. K. Allosteric transitions of glutamate dehydrogenase. Nature. 1968 Aug 31;219(5157):947–949. doi: 10.1038/219947a0. [DOI] [PubMed] [Google Scholar]
- OLSON J. A., ANFINSEN C. B. The crystallization and characterization of L-glutamic acid dehydrogenase. J Biol Chem. 1952 May;197(1):67–79. [PubMed] [Google Scholar]
- Price N. C., Radda G. K. Desensitization of glutamate dehydrogenase by reaction of tyrosne residues. Biochem J. 1969 Sep;114(2):419–427. doi: 10.1042/bj1140419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin M. M., Changeux J. P. On the nature of allosteric transitions: implications of non-exclusive ligand binding. J Mol Biol. 1966 Nov 14;21(2):265–274. doi: 10.1016/0022-2836(66)90097-0. [DOI] [PubMed] [Google Scholar]
- Stryer L. The interaction of a naphthalene dye with apomyoglobin and apohemoglobin. A fluorescent probe of non-polar binding sites. J Mol Biol. 1965 Sep;13(2):482–495. doi: 10.1016/s0022-2836(65)80111-5. [DOI] [PubMed] [Google Scholar]
- Sund H., Burchard W. Sedimentation coefficient and molecular weight of beef liver glutamate dehydrogenase at the microgram and the milligram level. Eur J Biochem. 1968 Nov;6(2):202–206. doi: 10.1111/j.1432-1033.1968.tb00438.x. [DOI] [PubMed] [Google Scholar]
- Thompson W., Yielding K. L. 8-Anilino naphthalene sulfonate binding as a probe for conformational changes induced in glutamate dehydrogenase by regulatory reagents. Arch Biochem Biophys. 1968 Aug;126(2):399–406. doi: 10.1016/0003-9861(68)90424-4. [DOI] [PubMed] [Google Scholar]
- YIELDING K. L., TOMKINS G. M., BITENSKY M. W., TALAL N. REAGENT-INDUCED CHANGES IN THE STRUCTURE AND CATALYTIC ACTIVITY OF GLUTAMIC DEHYDROGENASE. Can J Biochem. 1964 May;42:727–743. doi: 10.1139/o64-085. [DOI] [PubMed] [Google Scholar]


