Abstract
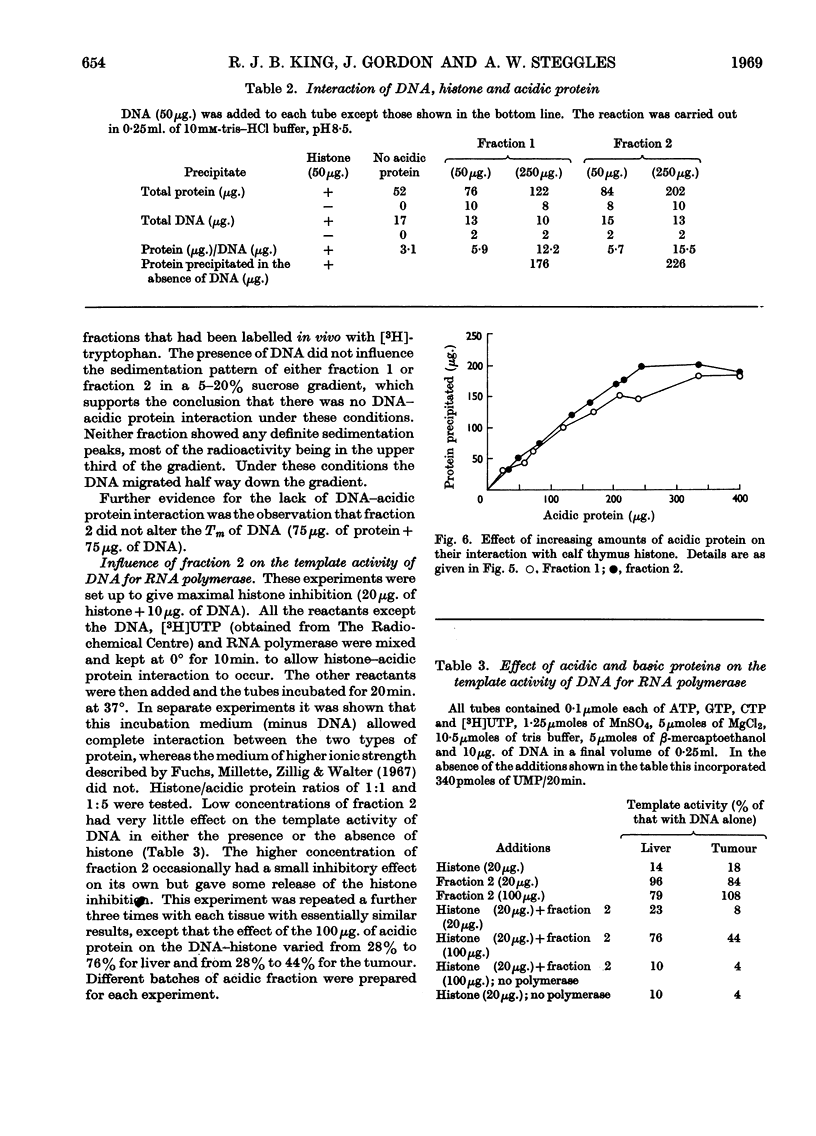
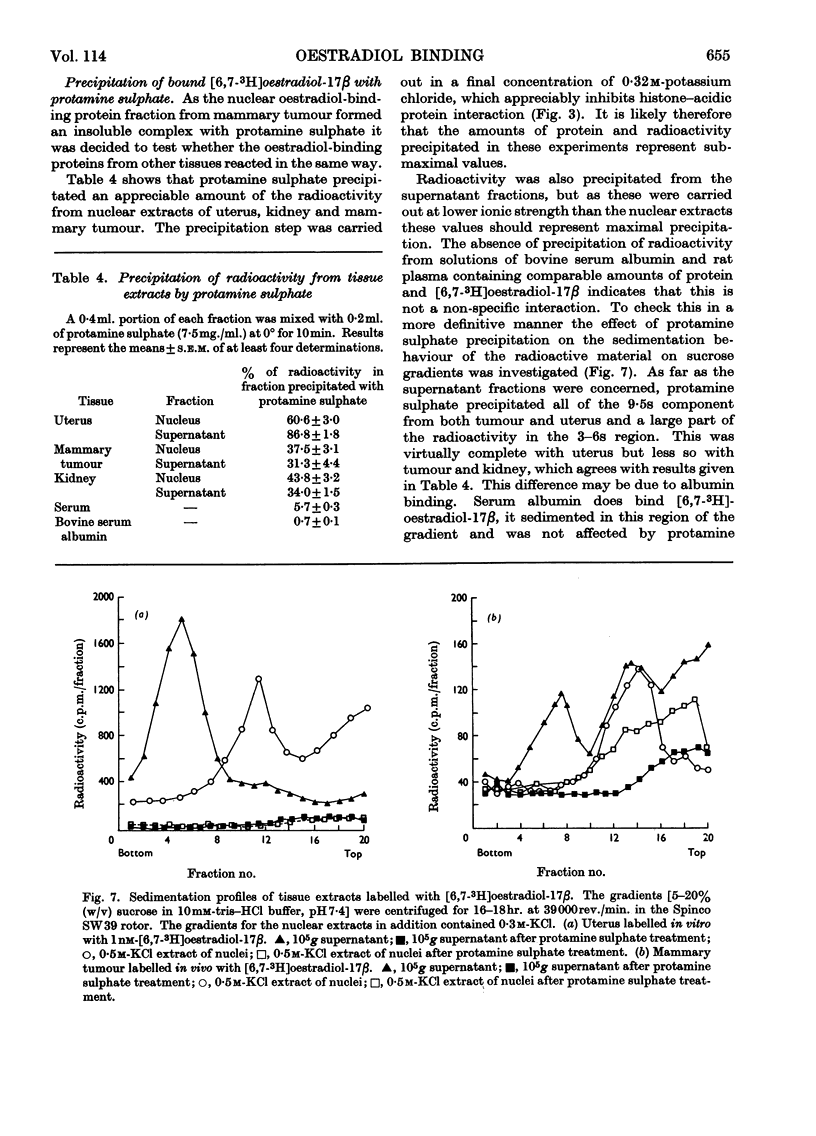
1. Additional evidence was obtained that the nuclear oestradiol-17β receptor is an acidic protein. Partial purification of the receptor protein was obtained by chromatography on hydroxyapatite and it contains protein-bound phosphate. 2. The nuclear `5s' and cytoplasmic `9·5s' and `5s' receptors from uterus, dimethylbenzanthracene-induced mammary adenocarcinoma and kidney are precipitated together with bound oestradiol-17β by protamine sulphate. This common property suggests that the nuclear and cytoplasmic receptors are related to each other. 3. The properties of two acidic protein fractions from both liver and dimethylbenzanthracene-induced mammary adenocarcinoma are described. Fraction 1 contains two major components and fraction 2 contains one component, as judged from polyacrylamide-gel electrophoresis. Fraction 2 contains RNA and both fractions contain protein-bound phosphate. 4. These fractions form insoluble complexes with calf thymus histone, protamine sulphate and poly-l-lysine. The formation of these complexes is markedly affected by ionic strength and pH. Ionization of both the ∈-amino group of lysine and carboxyl group are involved. RNA and DNA do not appear to be involved. The interaction is not affected by EDTA or 1mm-Na+, -K+, -Ca2+, -Mg2+ or -Mn2+. Per unit weight, whole histone has 4–5 times as many binding sites for the acidic proteins as the latter have for the former. 5. No convincing evidence was obtained for DNA–acidic protein interaction, but, as judged from precipitation experiments, there was competition between DNA and acidic protein for histone. 6. Relatively large amounts of acidic protein partly relieved the histone inhibition of the template activity of DNA for Escherichia coli RNA polymerase (EC 2.7.7.6).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLFREY V. G., LITTAU V. C., MIRSKY A. E. METHODS FOR THE PURIFICATION OF THYMUS NUCLEI AND THEIR APPLICATION TO STUDIES OF NUCLEAR PROTEIN SYNTHESIS. J Cell Biol. 1964 May;21:213–231. doi: 10.1083/jcb.21.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAILLIE L. A. Determination of liquid scientillation counting efficiency by pulse height shift. Int J Appl Radiat Isot. 1960 May;8:1–7. doi: 10.1016/0020-708x(60)90153-8. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrambach A., Reisfeld R. A., Wyckoff M., Zaccari J. A procedure for rapid and sensitive staining of protein fractionated by polyacrylamide gel electrophoresis. Anal Biochem. 1967 Jul;20(1):150–154. doi: 10.1016/0003-2697(67)90272-2. [DOI] [PubMed] [Google Scholar]
- Fuchse, Millette R. L., Zillig W., Walter G. Influence of salts on RNA synthesis by DNA-dependent RNA-polymerase from Escherichia coli. Eur J Biochem. 1967 Dec;3(2):183–193. doi: 10.1111/j.1432-1033.1967.tb19514.x. [DOI] [PubMed] [Google Scholar]
- GREENBAUM A. L., SLATER T. F. Studies on the particulate components of rat mammary gland. II. Changes in the levels of the nucleic acids of the mammary glands of rats during pregnancy, lactation and mammary involution. Biochem J. 1957 May;66(1):155–161. doi: 10.1042/bj0660155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGGINS C., BRIZIARELLI G., SUTTON H., Jr Rapid induction of mammary carcinoma in the rat and the influence of hormones on the tumors. J Exp Med. 1959 Jan 1;109(1):25–42. doi: 10.1084/jem.109.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton T. H. Control by estrogen of genetic transcription and translation. Binding to chromatin and stimulation of nucleolar RNA synthesis are primary events in the early estrogen action. Science. 1968 Aug 16;161(3842):649–661. doi: 10.1126/science.161.3842.649. [DOI] [PubMed] [Google Scholar]
- Jensen E. V., Suzuki T., Kawashima T., Stumpf W. E., Jungblut P. W., DeSombre E. R. A two-step mechanism for the interaction of estradiol with rat uterus. Proc Natl Acad Sci U S A. 1968 Feb;59(2):632–638. doi: 10.1073/pnas.59.2.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns E. W. Studies on histones. 7. Preparative methods for histone fractions from calf thymus. Biochem J. 1964 Jul;92(1):55–59. doi: 10.1042/bj0920055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J. B., Gordon J. The localization of [6,7-3H]oestradiol-17-beta in rat uterus. J Endocrinol. 1966 Apr;34(4):431–437. doi: 10.1677/joe.0.0340431. [DOI] [PubMed] [Google Scholar]
- King R. J. Fixation of steroids to receptors. Arch Anat Microsc Morphol Exp. 1967;56(3):570–583. [PubMed] [Google Scholar]
- King R. J., Gordon J. An attempt to isolate an oestradiol receptor from nuclei by adsorption on oestradiol-17-beta. J Endocrinol. 1968 Feb;40(2):195–204. doi: 10.1677/joe.0.0400195. [DOI] [PubMed] [Google Scholar]
- King R. J., Gordon J., Cowan D. M., Inman D. R. The intranuclear localization of [6,7-3H]-oestradiol-17-beta in dimethylbenzanthracene-induced rat mammary adenocarcinoma and other tissues. J Endocrinol. 1966 Oct;36(2):139–150. doi: 10.1677/joe.0.0360139. [DOI] [PubMed] [Google Scholar]
- King R. J., Gordon J. The association of [6,7-3H]oestradiol with a nuclear protein. J Endocrinol. 1967 Dec;39(4):533–542. doi: 10.1677/joe.0.0390533. [DOI] [PubMed] [Google Scholar]
- Kleinsmith L. J., Allfrey V. G., Mirsky A. E. Phosphoprotein metabolism in isolated lymphocyte nuclei. Proc Natl Acad Sci U S A. 1966 May;55(5):1182–1189. doi: 10.1073/pnas.55.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ptashne M., Hopkins N. The operators controlled by the lambda phage repressor. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1282–1287. doi: 10.1073/pnas.60.4.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards E. G., Coll J. A., Gratzer W. B. Disc electrophoresis of ribonucleic acid in polyacrylamide gels. Anal Biochem. 1965 Sep;12(3):452–471. doi: 10.1016/0003-2697(65)90212-5. [DOI] [PubMed] [Google Scholar]
- Rochefort H., Baulieu E. E. Récepteurs hormonaux: relations entre les "récepteurs" utérins de l'oestradiol, "8 S" cytoplasmique, et "4 S" cytoplasmique et nucléaire. C R Acad Sci Hebd Seances Acad Sci D. 1968 Aug 5;267(6):662–665. [PubMed] [Google Scholar]
- STEELE W. J., BUSCH H. STUDIES ON ACIDIC NUCLEAR PROTEINS OF THE WALKER TUMOR AND LIVER. Cancer Res. 1963 Sep;23:1153–1163. [PubMed] [Google Scholar]
- Smith J. A., King R. J., Meggitt B. F., Allen L. N. Biochemical studies on human and rat breast tissues. Br J Cancer. 1966 Jun;20(2):335–344. doi: 10.1038/bjc.1966.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toft D., Gorski J. A receptor molecule for estrogens: isolation from the rat uterus and preliminary characterization. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1574–1581. doi: 10.1073/pnas.55.6.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VELLE W., ENGEL L. L. ENZYMES FROM BOVINE PLACENTA AND SEMINAL VESICLES THAT OXIDIZE D(-)-1,2-PROPANEDIOL AND OTHER POLYOLS: THEIR POSSIBLE RELATION TO FRUCTOSE FORMATION. Endocrinology. 1964 Mar;74:429–439. doi: 10.1210/endo-74-3-429. [DOI] [PubMed] [Google Scholar]
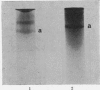
- Wang T. Y., Johns E. W. Study of the chromatin acidic proteins of rat liver: heterogeneity and complex formation with histones. Arch Biochem Biophys. 1968 Mar 20;124(1):176–183. doi: 10.1016/0003-9861(68)90318-4. [DOI] [PubMed] [Google Scholar]
- Wang T. Y. Restoration of histone-inhibited DNA-dependent RNA synthesis by acidic chromatin proteins. Exp Cell Res. 1968 Oct;53(1):288–291. doi: 10.1016/0014-4827(68)90377-7. [DOI] [PubMed] [Google Scholar]
- Warren J. C., Barker K. L. Cyclic variation in RNA synthesis potential in the uterus of the golden hamster. Biochim Biophys Acta. 1967 Apr 18;138(2):421–423. doi: 10.1016/0005-2787(67)90501-1. [DOI] [PubMed] [Google Scholar]
- YOUNG S., COWAN D. M., SUTHERLAND L. E. The histology of induced mammary tumours in rats. J Pathol Bacteriol. 1963 Apr;85:331–340. doi: 10.1002/path.1700850210. [DOI] [PubMed] [Google Scholar]