Abstract
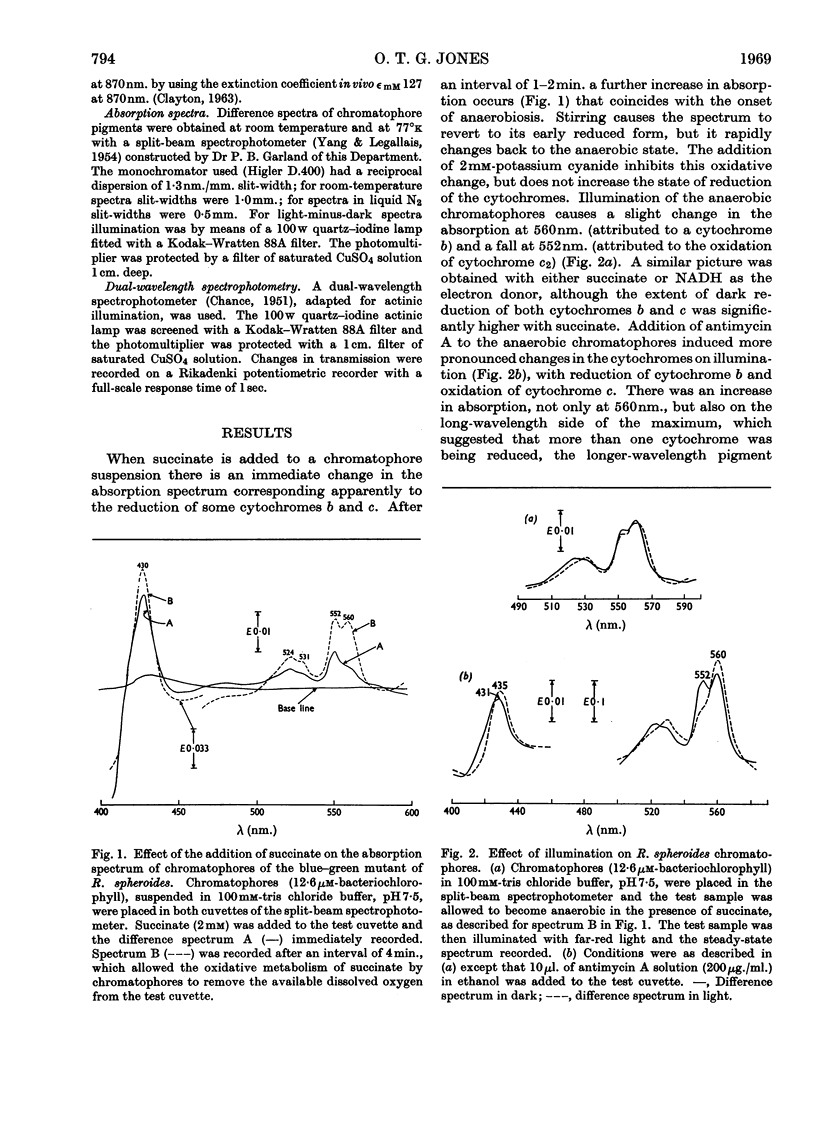
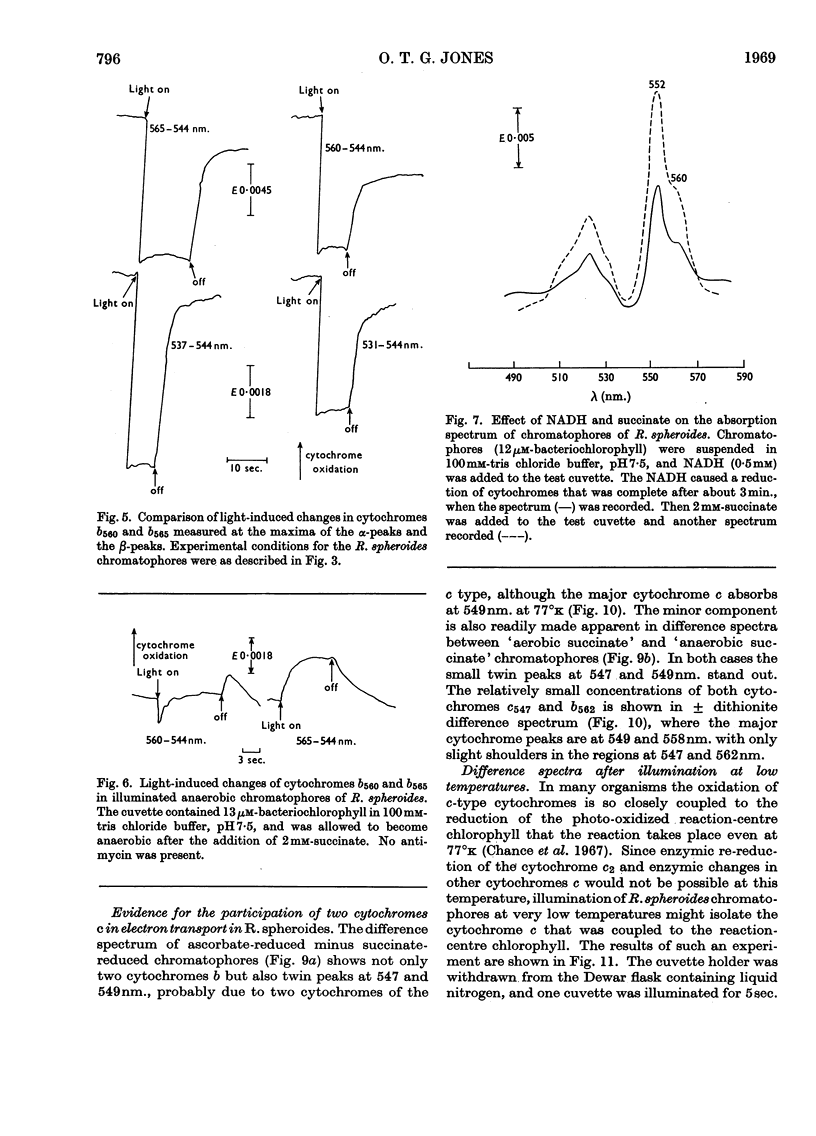
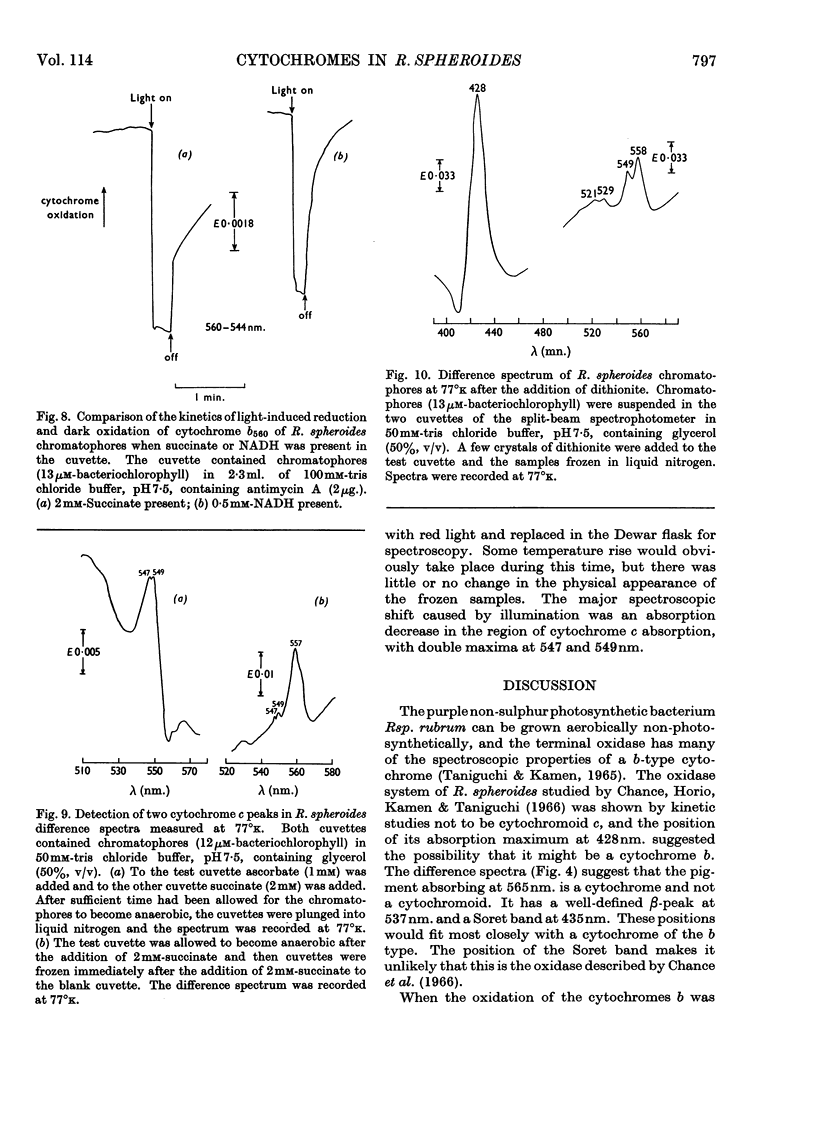
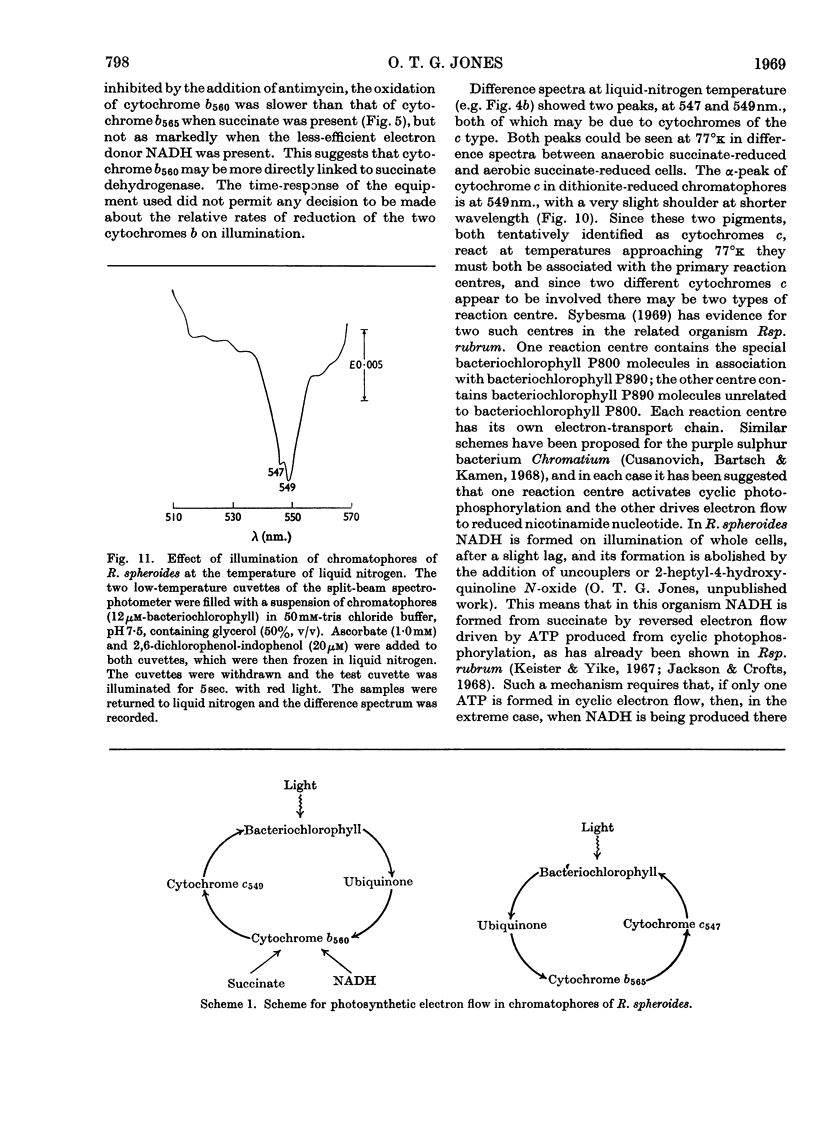
Illumination of chromatophore preparations from Rhodopseudomonas spheroides causes the oxidation of a cytochrome c and a slight oxidation of a cytochrome b with a maximum at 560nm. When illuminated in the presence of antimycin A the oxidation of cytochrome c was more pronounced and cytochrome b560 was reduced; the dark oxidation of cytochrome b560 was biphasic in the presence of succinate, but not in the presence of NADH, a less effective reductant. Split-beam spectroscopy showed that, in addition to the reduction of cytochrome b560, another pigment with maxima at 565 and 537nm. was reduced and was more rapidly oxidized in the dark than cytochrome b560. This pigment, tentatively identified as cytochrome b565, was also detected in spectra at 77°k, after brief illumination at room temperature; the maxima at 77°k were at 562 and 536nm. In the absence of antimycin A, light caused a transient reduction of cytochrome b565 and an oxidation of cytochrome b560. Dark oxidation of b565 was rapid, even in the presence of antimycin A and succinate. Difference spectra, at 77°k, of ascorbate-reduced minus succinate-reduced chromatophores or of anaerobic succinate-reduced minus aerobic succinate-reduced chromatophores suggested that two cytochromes c were present, with maxima at 547 and 549nm. When chromatophores frozen at 77°k were illuminated both these cytochromes c were oxidized, indicating a close association with the photochemical reaction centre. A scheme involving two reaction centres is proposed to explain these results.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chance B., DeVault D., Hildreth W. W., Parson W. W., Nishimura M. Early chemical events in photosynthesis: kinetics of oxidation of cytochromes of types c or f in cells, chloroplasts, and chromatophores. Brookhaven Symp Biol. 1966;19:115–131. [PubMed] [Google Scholar]
- Chance B., Horio T., Kamen M. D., Taniguchi S. Kinetic studies on the oxidase systems of photosynthetic bacteria. Biochim Biophys Acta. 1966 Jan 4;112(1):1–7. doi: 10.1016/s0926-6585(96)90002-3. [DOI] [PubMed] [Google Scholar]
- Clayton R. K. The bacterial photosynthetic reaction center. Brookhaven Symp Biol. 1966;19:62–70. [PubMed] [Google Scholar]
- Cusanovich M. A., Bartsch R. G., Kamen M. D. Light-induced electron transport in Chromatium strain D. II. Light-induced absorbance changes in Chromatium chromatophores. Biochim Biophys Acta. 1968 Feb 12;153(2):397–417. doi: 10.1016/0005-2728(68)90083-2. [DOI] [PubMed] [Google Scholar]
- Jackson J. B., Crofts A. R. Energy-linked reduction of nicotinamide adenine dinucleotides in cells of Rhodospirillum rubrum. Biochem Biophys Res Commun. 1968 Sep 30;32(6):908–915. doi: 10.1016/0006-291x(68)90113-7. [DOI] [PubMed] [Google Scholar]
- KAMEN M. D., VERNON L. P. Comparative studies on bacterial cytochromes. Biochim Biophys Acta. 1955 May;17(1):10–22. doi: 10.1016/0006-3002(55)90315-2. [DOI] [PubMed] [Google Scholar]
- KUNTZ I. D., Jr, LOACH P. A., CALVIN M. ABSORPTION CHANGES IN BACTERIAL CHROMATOPHORES. Biophys J. 1964 May;4:227–249. doi: 10.1016/s0006-3495(64)86779-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keister D. L., Yike N. J. Energy-linked reactions in photosynthetic bacteria. I. Succinatelinked ATP-driven NAD reduction by Rhodospirillum rubrum chromatophores. Arch Biochem Biophys. 1967 Aug;121(2):415–422. doi: 10.1016/0003-9861(67)90095-1. [DOI] [PubMed] [Google Scholar]
- MORET V., PINAMONTI S., FORNASARI E. Polarographic study on the redox potential of ubiquinones. Biochim Biophys Acta. 1961 Dec 9;54:381–383. doi: 10.1016/0006-3002(61)90388-2. [DOI] [PubMed] [Google Scholar]
- Morita S. Evidence for three photochemical systems in Chromatium D. Biochim Biophys Acta. 1968 Jan 15;153(1):241–247. doi: 10.1016/0005-2728(68)90166-7. [DOI] [PubMed] [Google Scholar]
- NISHIMURA M. Studies on the electron-transfer systems in photosynthetic bacteria. II. The effect of heptylhydroxyquinoline-N-oxide and antimycin A on the photosynthetic and respiratory electron-transfer systems. Biochim Biophys Acta. 1963 Jan 15;66:17–21. doi: 10.1016/0006-3002(63)91163-6. [DOI] [PubMed] [Google Scholar]
- SISTROM W. R. A requirement for sodium in the growth of Rhodopseudomonas spheroides. J Gen Microbiol. 1960 Jun;22:778–785. doi: 10.1099/00221287-22-3-778. [DOI] [PubMed] [Google Scholar]
- Sybesma C. Light-induced reactions of P890 and P800 in the purple photosynthetic bacterium Rhodospirillum rubrum. Biochim Biophys Acta. 1969 Jan 14;172(1):177–179. doi: 10.1016/0005-2728(69)90104-2. [DOI] [PubMed] [Google Scholar]
- TANIGUCHI S., KAMEN M. D. THE OXIDASE SYSTEM OF HETEROTROPHICALLY-GROWN RHODOSPIRILLUM RUBRUM. Biochim Biophys Acta. 1965 Mar 22;96:395–428. doi: 10.1016/0005-2787(65)90560-5. [DOI] [PubMed] [Google Scholar]
- Vernon L. P. Photochemical and electron transport reactions of bacterial photosynthesis. Bacteriol Rev. 1968 Sep;32(3):243–261. doi: 10.1128/br.32.3.243-261.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]