Abstract
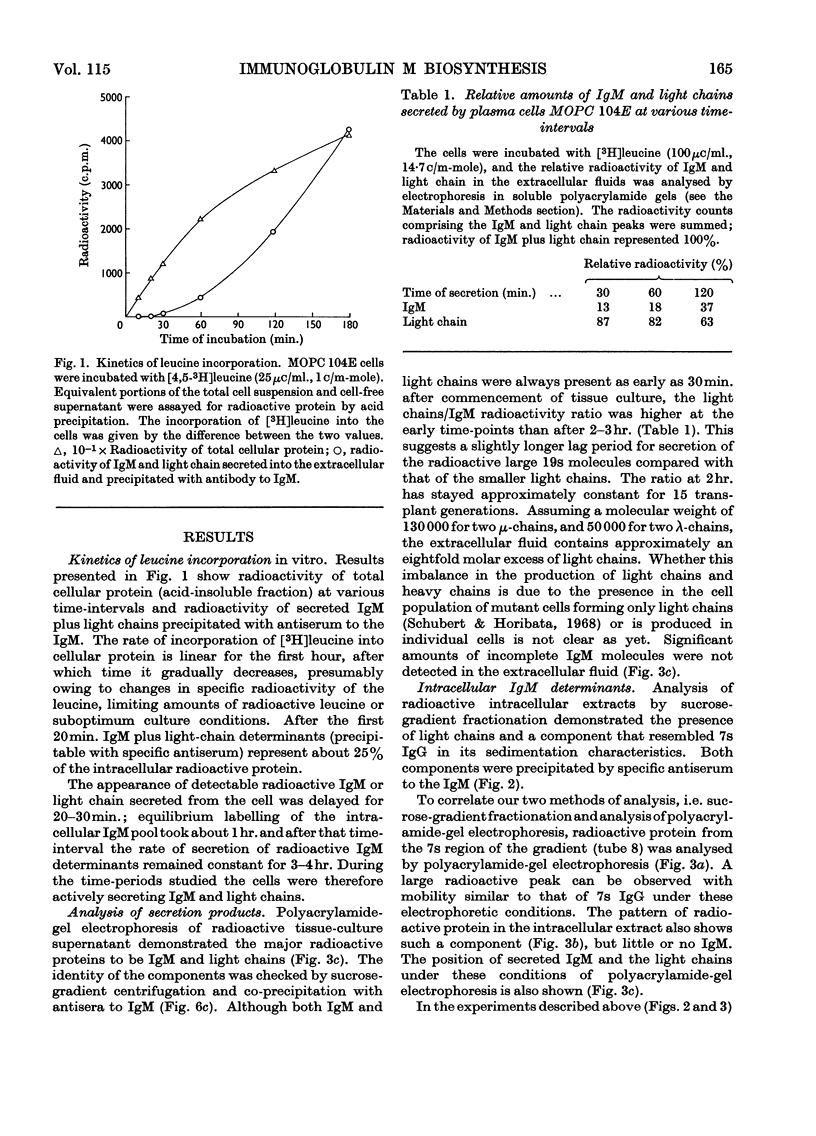
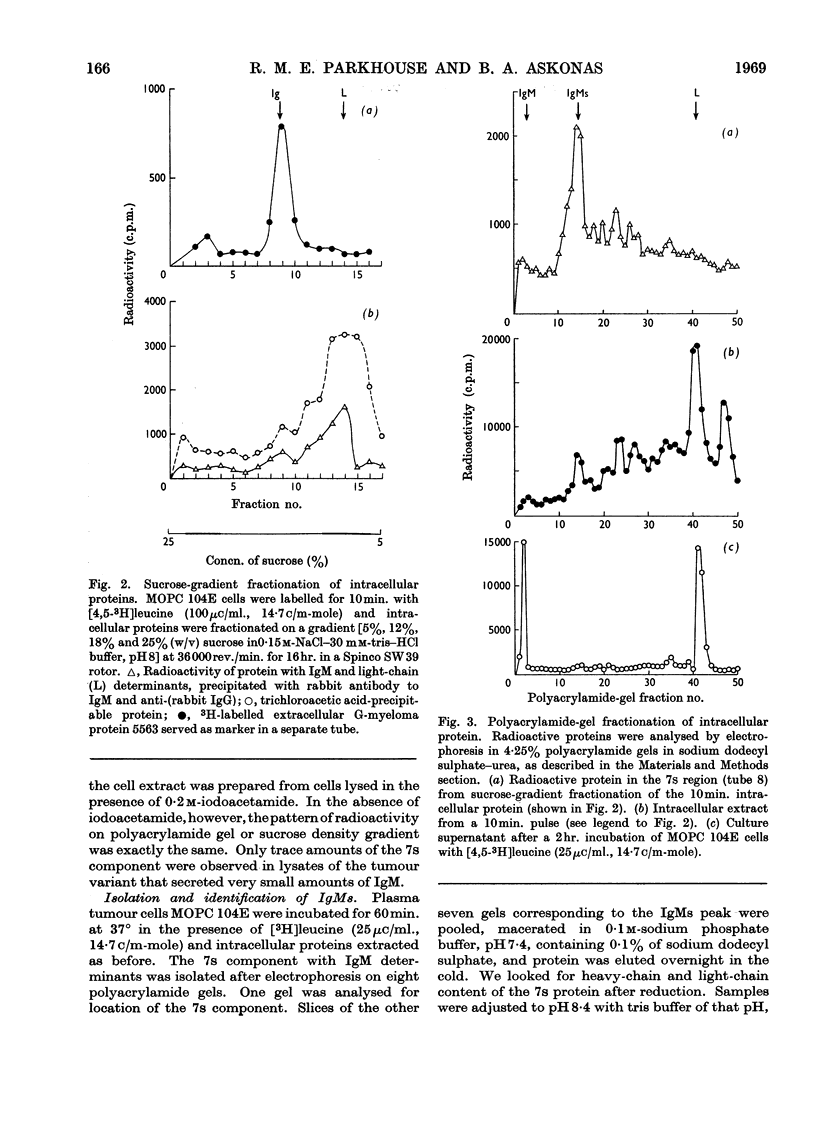
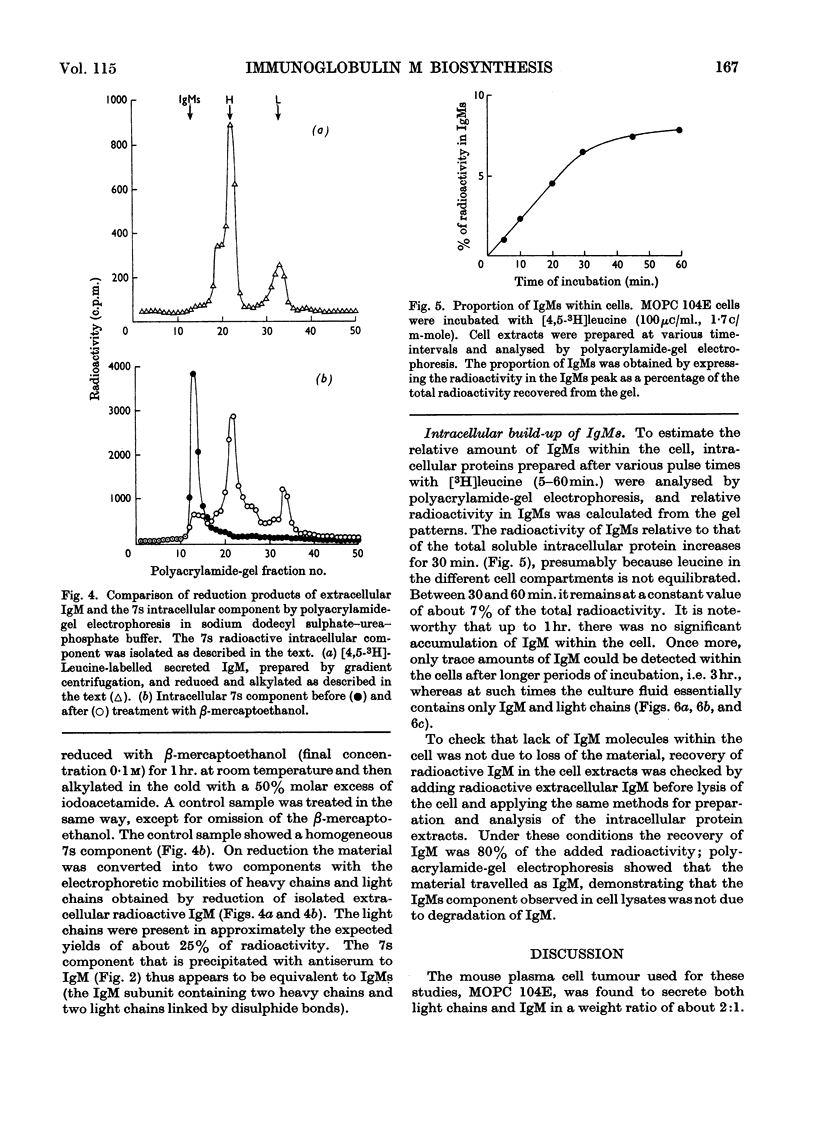
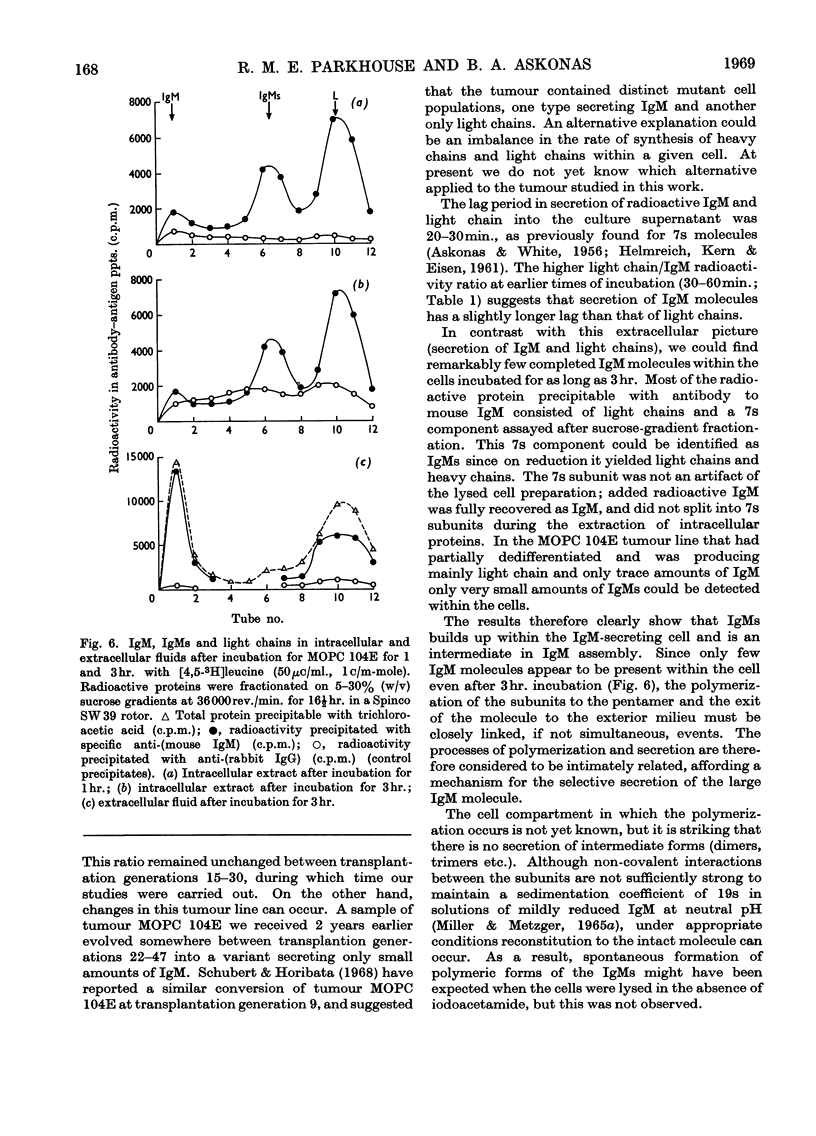
Immunoglobulin M biosynthesis was studied with mouse plasma cell tumour MOPC 104E as a model system. Cell suspensions prepared from solid tumours were incubated in vitro with tritiated leucine; the radioactivity incorporated into intracellular and secreted proteins was analysed by polyacrylamide-gel electrophoresis, sucrose-density-gradient centrifugation and precipitation with rabbit antiserum specific for the macroglobulin. The tumour was found to secrete immunoglobulin M and light chains in a 1:2 weight ratio, with lag periods of 20–30min. Within the cells there was a 7s component precipitable with specific antiserum to the macroglobulin that was shown to consist of heavy and light chains. This 7s subunit of the macroglobulin appeared to accumulate in the intracellular environment, so that even after long periods of incubation (3hr.) no more than trace amounts of fully assembled 19s molecules could be detected in cell lysates. Polymerization of the subunits into the pentamer therefore appears to take place shortly before, or simultaneously with, secretion of the molecules.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASKONAS B. A., WHITE R. G. Sites of antibody production in the guinea-pig; the relation between in vitro synthesis of anti-ovalbumin and gamma-globulin and distribution of antibody-containing plasma cells. Br J Exp Pathol. 1956 Feb;37(1):61–74. [PMC free article] [PubMed] [Google Scholar]
- Abel C. A., Grey H. M. Carboxy-terminal amino acids of gamma-A and gamma-M heavy chains. Science. 1967 Jun 23;156(3782):1609–1610. doi: 10.1126/science.156.3782.1609. [DOI] [PubMed] [Google Scholar]
- Ashman R. F., Metzger H. A Waldenström macroglobulin which binds nitrophenyl ligands. J Biol Chem. 1969 Jun 25;244(12):3405–3414. [PubMed] [Google Scholar]
- Borun T. W., Scharff M. D., Robbins E. Preparation of mammalian polyribosomes with the detergent Nonidet P-40. Biochim Biophys Acta. 1967 Nov 21;149(1):302–304. doi: 10.1016/0005-2787(67)90715-0. [DOI] [PubMed] [Google Scholar]
- Brownstone A. D. A versatile system for preparative electrophoresis in acrylamide gel. Anal Biochem. 1969 Jan;27(1):25–46. doi: 10.1016/0003-2697(69)90216-4. [DOI] [PubMed] [Google Scholar]
- Clem I. W., De Boutaud F., Sigel M. M. Phylogeny of immunoglobulin structure and function. II. Immunoglobulins of the nurse shark. J Immunol. 1967 Dec;99(6):1226–1235. [PubMed] [Google Scholar]
- Clem L. W., Small P. A., Jr Phylogeny of immunoglobulin structure and function. I. Immunoglobulins of the lemon shark. J Exp Med. 1967 May 1;125(5):893–920. doi: 10.1084/jem.125.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Milstein C. Structure and biological properties of immunoglobulins. Adv Immunol. 1967;7:1–89. doi: 10.1016/s0065-2776(08)60126-1. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F., Singer S. J., Metzger H. Evolution of immunoglobulin polypeptide chains: carboxy-terminal of an IgM heavy chain. Science. 1966 Dec 23;154(3756):1561–1562. doi: 10.1126/science.154.3756.1561. [DOI] [PubMed] [Google Scholar]
- FLEISCHMAN J. B., PORTER R. R., PRESS E. M. THE ARRANGEMENT OF THE PEPTIDE CHAINS IN GAMMA-GLOBULIN. Biochem J. 1963 Aug;88:220–228. doi: 10.1042/bj0880220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELMREICH E., KERN M., EISEN H. N. The secretion of antibody by isolated lymph node cells. J Biol Chem. 1961 Feb;236:464–473. [PubMed] [Google Scholar]
- KILLANDER J. SEPARATION OF HUMAN IMMUNOGLOBULINS BY GEL FILTRATION AND ZONE ELECTROPHORESIS. Acta Soc Med Ups. 1963;68:230–244. [PubMed] [Google Scholar]
- Klein F., Mattern P., Radema H., Van Zwet T. L. Slowly sedimenting serum components reacting with anti-IgM sera. Immunology. 1967 Dec;13(6):641–647. [PMC free article] [PubMed] [Google Scholar]
- Lamm M. E., Small P. A., Jr Polypeptide chain structure of rabbit immunoglobulins. II. gamma-M-immunoglobulin. Biochemistry. 1966 Jan;5(1):267–276. doi: 10.1021/bi00865a035. [DOI] [PubMed] [Google Scholar]
- MILLER F., METZGER H. CHARACTERIZATION OF A HUMAN MACROGLOBULIN. I. THE MOLECULAR WEIGHT OF ITS SUBUNIT. J Biol Chem. 1965 Aug;240:3325–3333. [PubMed] [Google Scholar]
- Marchalonis J., Edelman G. M. Phylogenetic origins of antibody structure. I. Multichain structure of immunoglobulins in the smooth dogfish (Mustelus canis). J Exp Med. 1965 Sep 1;122(3):601–618. doi: 10.1084/jem.122.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntimif K. R., Asofsky R. M., Potter M., Kuff E. L. Macroglobulin-Producing Plasma-Cell Tumor in Mice: Identification of a New Light Chain. Science. 1965 Oct 15;150(3694):361–363. doi: 10.1126/science.150.3694.361. [DOI] [PubMed] [Google Scholar]
- Mihaesco E., Mihaesco C. Carboxyterminal residues of the Fc fragment from human IgM. Biochem Biophys Res Commun. 1968 Dec 9;33(5):869–873. doi: 10.1016/0006-291x(68)90242-8. [DOI] [PubMed] [Google Scholar]
- Miller F., Metzger H. Characterization of a human macroglobulin. II. Distribution of the disulfide bonds. J Biol Chem. 1965 Dec;240(12):4740–4745. [PubMed] [Google Scholar]
- Morris J. E., Inman F. P. Isolation of the monomeric subunit of immunoglobulin M with its interchain disulfide bonds intact. Biochemistry. 1968 Aug;7(8):2851–2857. doi: 10.1021/bi00848a022. [DOI] [PubMed] [Google Scholar]
- ROTHFIELD N. F., FRANGIONE B., FRANKLIN E. C. SLOWLY SEDIMENTING MERCAPTOETHANOL-RESISTANT ANTINUCLEAR FACTORS RELATED ANTIGENICALLY TO M IMMUNOGLOBULINS (GAMMA-1M-GLOBULIN) IN PATIENTS WITH SYSTEMIC LUPUS ERYTHEMATOSUS. J Clin Invest. 1965 Jan;44:62–72. doi: 10.1172/JCI105127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sela M. Antibodies: Shape, homogeneity, and valency. FEBS Lett. 1968 Aug;1(2):83–85. doi: 10.1016/0014-5793(68)80025-0. [DOI] [PubMed] [Google Scholar]
- Solomon A., Kunkel H. G. A "monoclonal" type, low molecular weight protein related to gamma-M-macroglobulins. Am J Med. 1967 Jun;42(6):958–967. doi: 10.1016/0002-9343(67)90076-9. [DOI] [PubMed] [Google Scholar]
- Stobo J. D., Tomasi T. B. A Low Molecular Weight Immunoglobulin Antigenically Related to 19 S IgM. J Clin Invest. 1967 Aug;46(8):1329–1337. doi: 10.1172/JCI105625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr, Darnell J. E., Jr Evidence for virus-specific noncapsid proteins in poliovirus-infected HeLa cells. Proc Natl Acad Sci U S A. 1965 Aug;54(2):505–513. doi: 10.1073/pnas.54.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Deutsch H. F. Dissociation, reaggregation, and subunit structure studies of some human gamma-M-globulins. J Biol Chem. 1967 Jun 10;242(11):2725–2738. [PubMed] [Google Scholar]


