Abstract
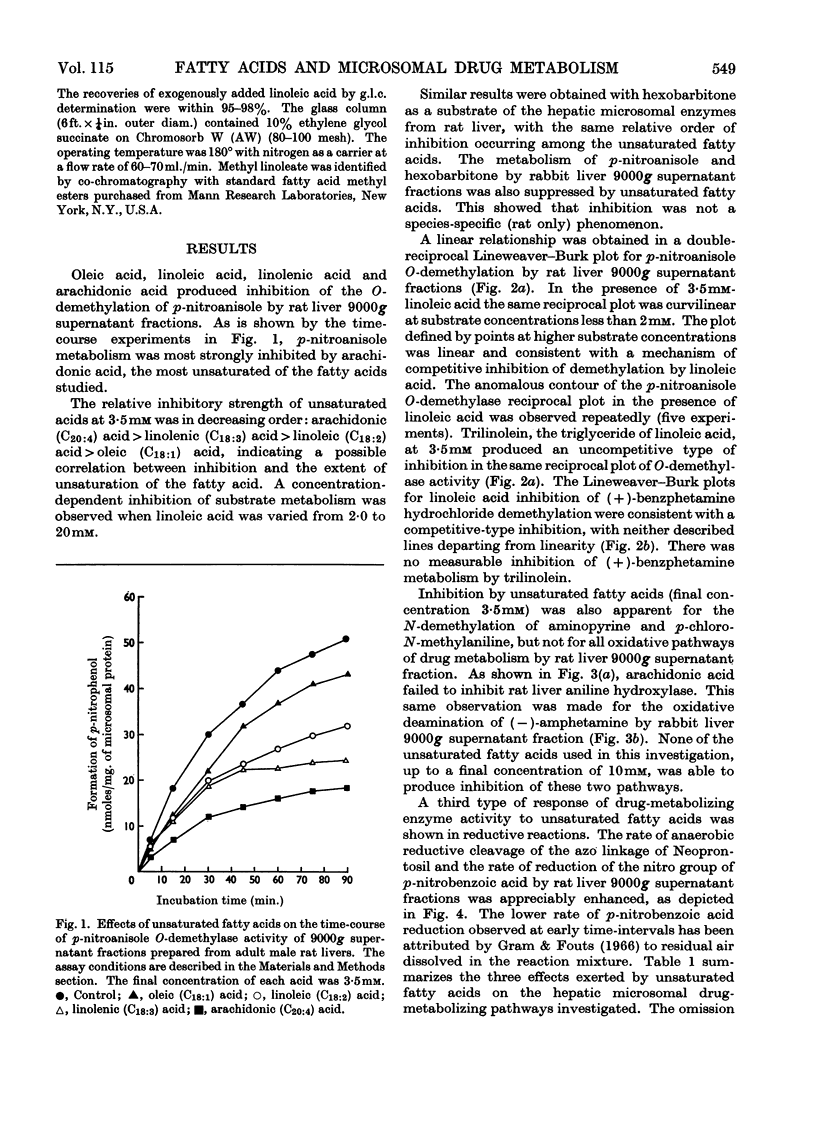
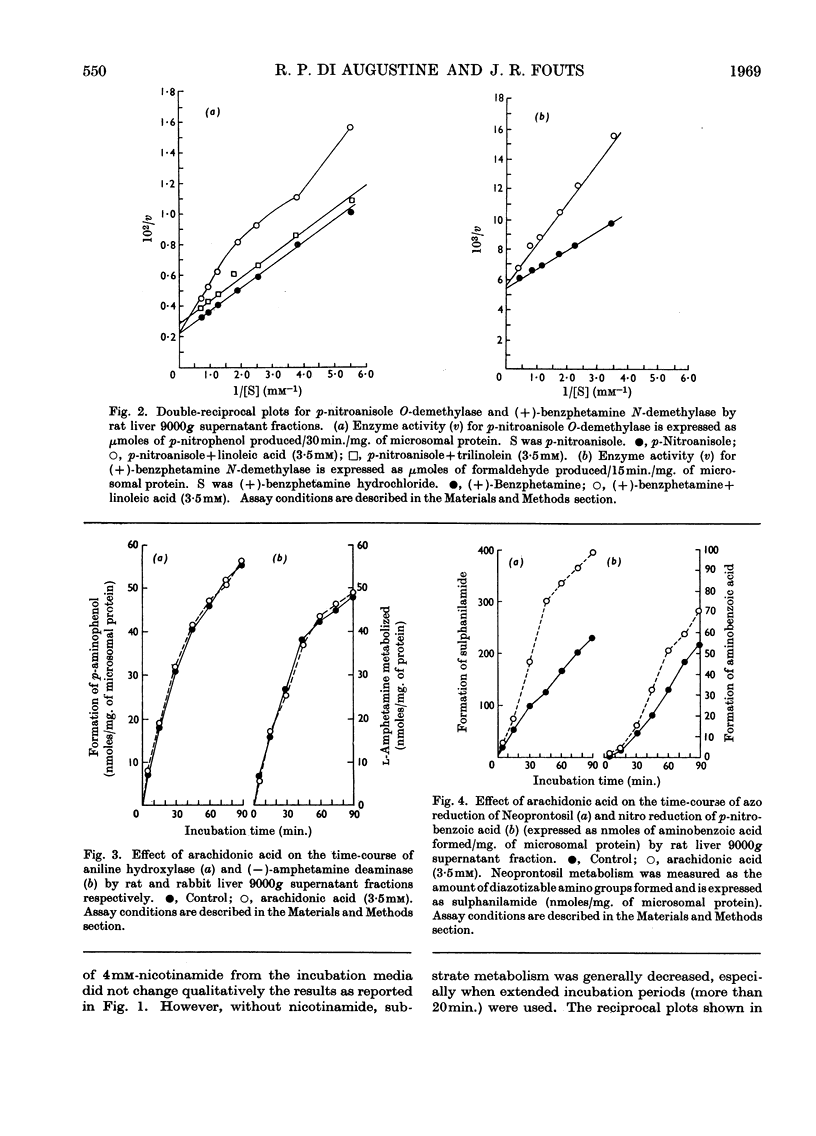
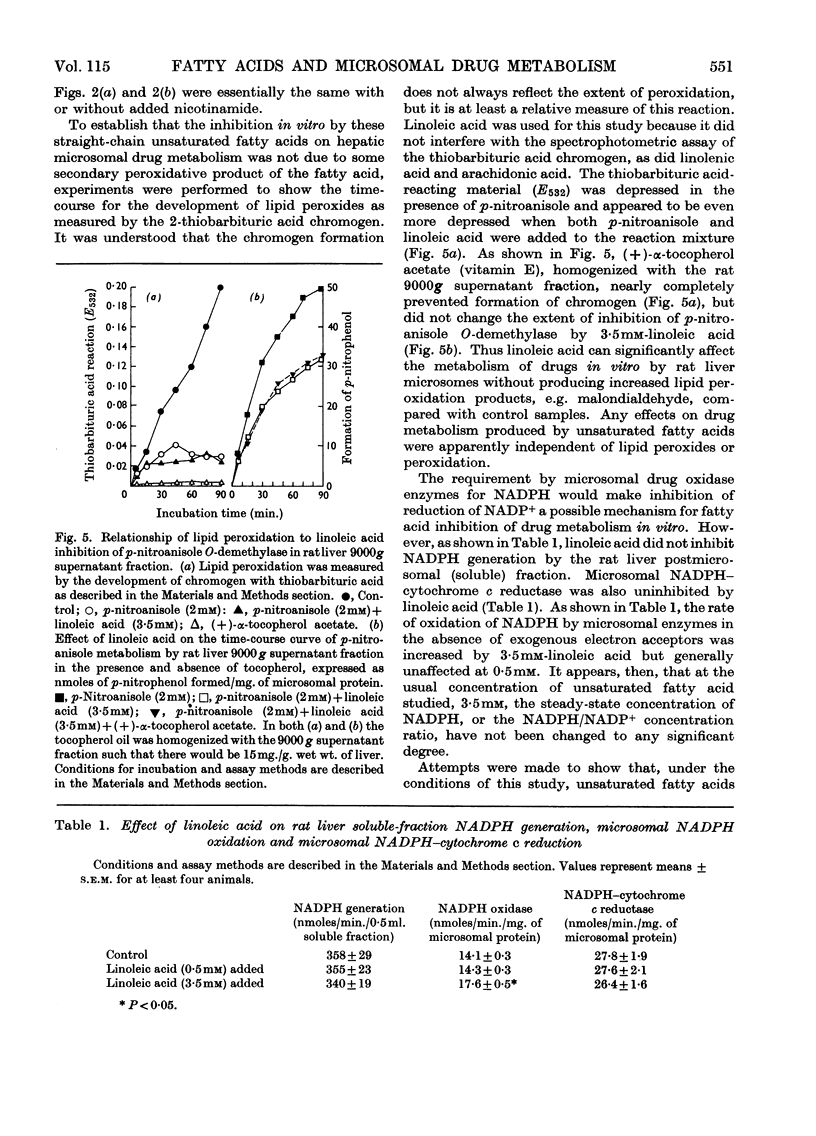
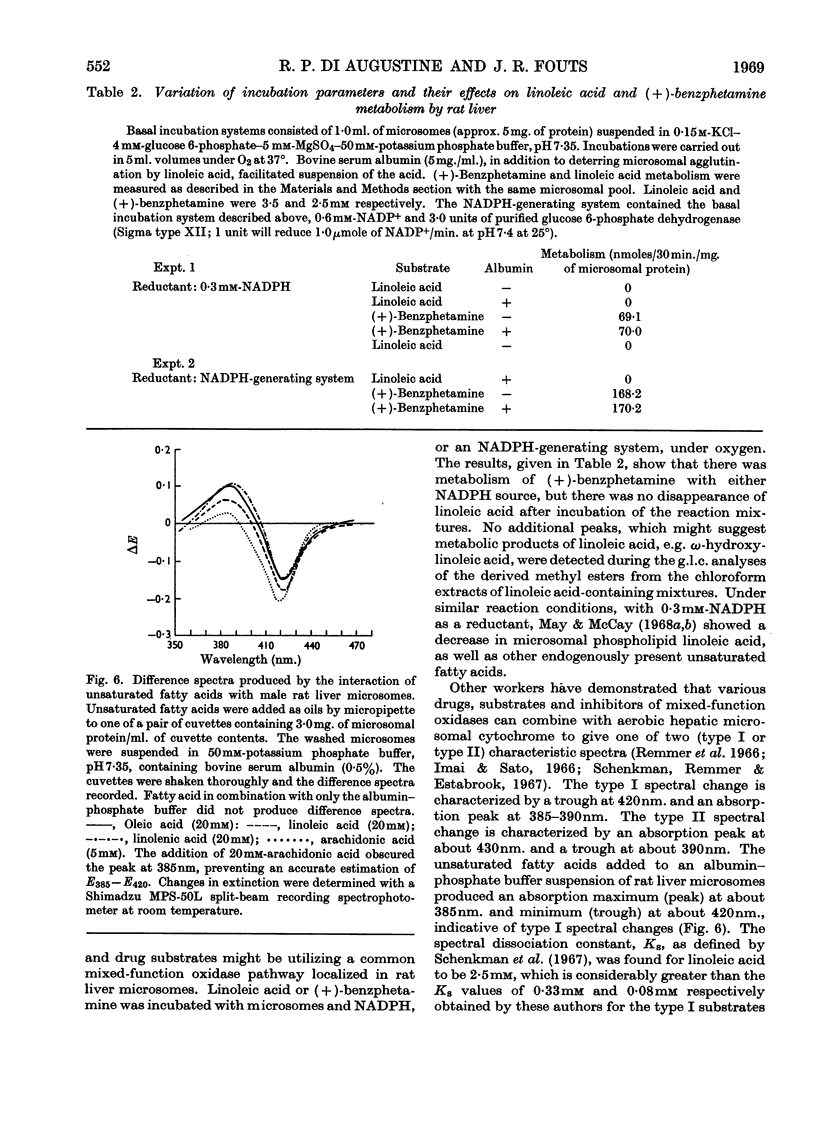
1. The effects of unsaturated fatty acids on drug-metabolizing enzymes in vitro were measured by using rat and rabbit hepatic 9000g supernatant fractions. 2. Unsaturated fatty acids inhibited the hepatic microsomal metabolism of `type I' drugs with inhibition increasing with unsaturation: arachidonic acid>linolenic acid>linoleic acid>oleic acid. Inhibition was independent of lipid peroxidation. Linoleic acid competitively inhibited the microsomal O-demethylation of p-nitroanisole and the N-demethylation of (+)-benzphetamine. 3. The hepatic microsomal metabolism of `type II' substrates, aniline and (−)-amphetamine, was not affected by unsaturated fatty acids. 4. The rate of reduction of p-nitrobenzoic acid and Neoprontosil was accelerated by unsaturated fatty acids. 5. Linoleic acid up to 3·5mm did not decelerate the generation of NADPH by rat liver soluble fraction, nor the activity of NADPH–cytochrome c reductase of rat liver microsomes. Hepatic microsomal NADPH oxidase activity was slightly enhanced by added linoleic acid. 6. No measurable disappearance of exogenously added linoleic acid occurred when this fatty acid was incubated with rat liver microsomes and an NADPH source. 7. The unsaturated fatty acids used in this study produced type I spectra when added to rat liver microsomes, and affected several microsomal enzyme activities in a manner characteristic of type I ligands.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELROD J. Studies on sympathomimetic amines. II. The biotransformation and physiological disposition of d-amphetamine, d-p-hydroxyamphetamine and d-methamphetamine. J Pharmacol Exp Ther. 1954 Mar;110(3):315–326. [PubMed] [Google Scholar]
- COCHIN J., AXELROD J. Biochemical and pharmacological changes in the rat following chronic administration of morphine nalorphine and normorphine. J Pharmacol Exp Ther. 1959 Feb;125(2):105–110. [PubMed] [Google Scholar]
- Das M. L., Orrenius S., Ernster L. On the fatty acid and hydrocarbon hydroxylation in rat liver microsomes. Eur J Biochem. 1968 May;4(4):519–523. doi: 10.1111/j.1432-1033.1968.tb00243.x. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- FOUTS J. R., BRODIE B. B. The enzymatic reduction of chloramphenicol, p-nitrobenzoic acid and other aromatic nitro compounds in mammals. J Pharmacol Exp Ther. 1957 Feb;119(2):197–207. [PubMed] [Google Scholar]
- FOUTS J. R., KAMM J. J., BRODIE B. B. Enzymatic reduction of prontosil and other azo dyes. J Pharmacol Exp Ther. 1957 Jul;120(3):291–300. [PubMed] [Google Scholar]
- GILLETTE J. R., BRODIE B. B., LA DU B. N. The oxidation of drugs by liver microsomes: on the role of TPNH and oxygen. J Pharmacol Exp Ther. 1957 Apr;119(4):532–540. [PubMed] [Google Scholar]
- Gigon P. L., Gram T. E., Gillette J. R. Studies on the rate of reduction of hepatic microsomal cytochrome P-450 by reduced nicotinamide adenine dinucleotide phosphate: effect of drug substrates. Mol Pharmacol. 1969 Mar;5(2):109–122. [PubMed] [Google Scholar]
- Gram T. E., Fouts J. R. Time course differences in the metabolism of drugs by hepatic microsomes from rats, rabbits and mice. J Pharmacol Exp Ther. 1966 Jun;152(3):363–371. [PubMed] [Google Scholar]
- Gram T. E., Rogers L. A., Fouts J. R. Further studies on the metabolism of drugs by subfractions of hepatic microsomes. J Pharmacol Exp Ther. 1967 Mar;155(3):479–493. [PubMed] [Google Scholar]
- HUNTER F. E., Jr, GEBICKI J. M., HOFFSTEN P. E., WEINSTEIN J., SCOTT A. Swelling and lysis of rat liver mitochondria induced by ferrous ions. J Biol Chem. 1963 Feb;238:828–835. [PubMed] [Google Scholar]
- Imai Y., Sato R. Substrate interaction with hydroxylase system in liver microsomes. Biochem Biophys Res Commun. 1966 Mar 22;22(6):620–626. doi: 10.1016/0006-291x(66)90191-4. [DOI] [PubMed] [Google Scholar]
- Kuntzman R., Lawrence D., Conney A. H. Michaelis constants for the hydroxylation of steroid hormones and drugs by rat liver microsomes. Mol Pharmacol. 1965 Sep;1(2):163–167. [PubMed] [Google Scholar]
- Kupfer D., Bruggeman L. L. Determination of enzymic demethylation of p-chloro-N-methylaniline. Assay of aniline and p-chloroaniline. Anal Biochem. 1966 Dec;17(3):502–512. doi: 10.1016/0003-2697(66)90185-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lu A. Y., Coon M. J. Role of hemoprotein P-450 in fatty acid omega-hydroxylation in a soluble enzyme system from liver microsomes. J Biol Chem. 1968 Mar 25;243(6):1331–1332. [PubMed] [Google Scholar]
- MORRISON W. R., SMITH L. M. PREPARATION OF FATTY ACID METHYL ESTERS AND DIMETHYLACETALS FROM LIPIDS WITH BORON FLUORIDE--METHANOL. J Lipid Res. 1964 Oct;5:600–608. [PubMed] [Google Scholar]
- May H. E., McCay P. B. Reduced triphosphopyridine nucleotide oxidase-catalyzed alterations of membrane phospholipids. I. Nature of the lipid alterations. J Biol Chem. 1968 May 10;243(9):2288–2295. [PubMed] [Google Scholar]
- May H. E., McCay P. B. Reduced triphosphopyridine nucleotide oxidase-catalyzed alterations of membrane phospholipids. II. Enzymic properties and stoichiometry. J Biol Chem. 1968 May 10;243(9):2296–2305. [PubMed] [Google Scholar]
- McLean A. E. Effect of hexane and carbon tetrachloride on microsomal cytochrome (P450). Biochem Pharmacol. 1967 Oct;16(10):2030–2033. doi: 10.1016/0006-2952(67)90317-6. [DOI] [PubMed] [Google Scholar]
- NETTER K. J., SEIDEL G. AN ADAPTIVELY STIMULATED O-DEMETHYLATING SYSTEM IN RAT LIVER MICROSOMES AND ITS KINETIC PROPERTIES. J Pharmacol Exp Ther. 1964 Oct;146:61–65. [PubMed] [Google Scholar]
- PHILLIPS A. H., LANGDON R. G. Hepatic triphosphopyridine nucleotide-cytochrome c reductase: isolation, characterization, and kinetic studies. J Biol Chem. 1962 Aug;237:2652–2660. [PubMed] [Google Scholar]
- PREISS B., BLOCH K. OMEGA-OXIDATION OF LONG CHAIN FATTY ACIDS IN RAT LIVER. J Biol Chem. 1964 Jan;239:85–88. [PubMed] [Google Scholar]
- Remmer H., Schenkman J., Estabrook R. W., Sasame H., Gillette J., Narasimhulu S., Cooper D. Y., Rosenthal O. Drug interaction with hepatic microsomal cytochrome. Mol Pharmacol. 1966 Mar;2(2):187–190. [PubMed] [Google Scholar]
- Roberts R. J., Plaa G. L. Effect of norethandrolone, acetohexamide, and Enovid on alpha-naphthylisothiocyanate-induced hyperbilirubinemia and cholestasis. Biochem Pharmacol. 1966 Mar;15(3):333–341. doi: 10.1016/0006-2952(66)90304-2. [DOI] [PubMed] [Google Scholar]
- Sasame H. A., Gillette J. R. Studies on the relationship between the effects of various substances on absorption spectrum of cytochrome P-450 and the reduction of p-nitrobenzoate by mouse liver microsomes. Mol Pharmacol. 1969 Mar;5(2):123–130. [PubMed] [Google Scholar]
- Schenkman J. B., Remmer H., Estabrook R. W. Spectral studies of drug interaction with hepatic microsomal cytochrome. Mol Pharmacol. 1967 Mar;3(2):113–123. [PubMed] [Google Scholar]
- Solomon H. M., Schrogie J. J., Williams D. The displacement of phenylbutazone-14C and warfarin-14C from human albumin by various drugs and fatty acids. Biochem Pharmacol. 1968 Jan;17(1):143–151. doi: 10.1016/0006-2952(68)90166-4. [DOI] [PubMed] [Google Scholar]
- Tephly T. R., Mannering G. J. Inhibition of drug metabolism. V. Inhibition of drug metabolism by steroids. Mol Pharmacol. 1968 Jan;4(1):10–14. [PubMed] [Google Scholar]


