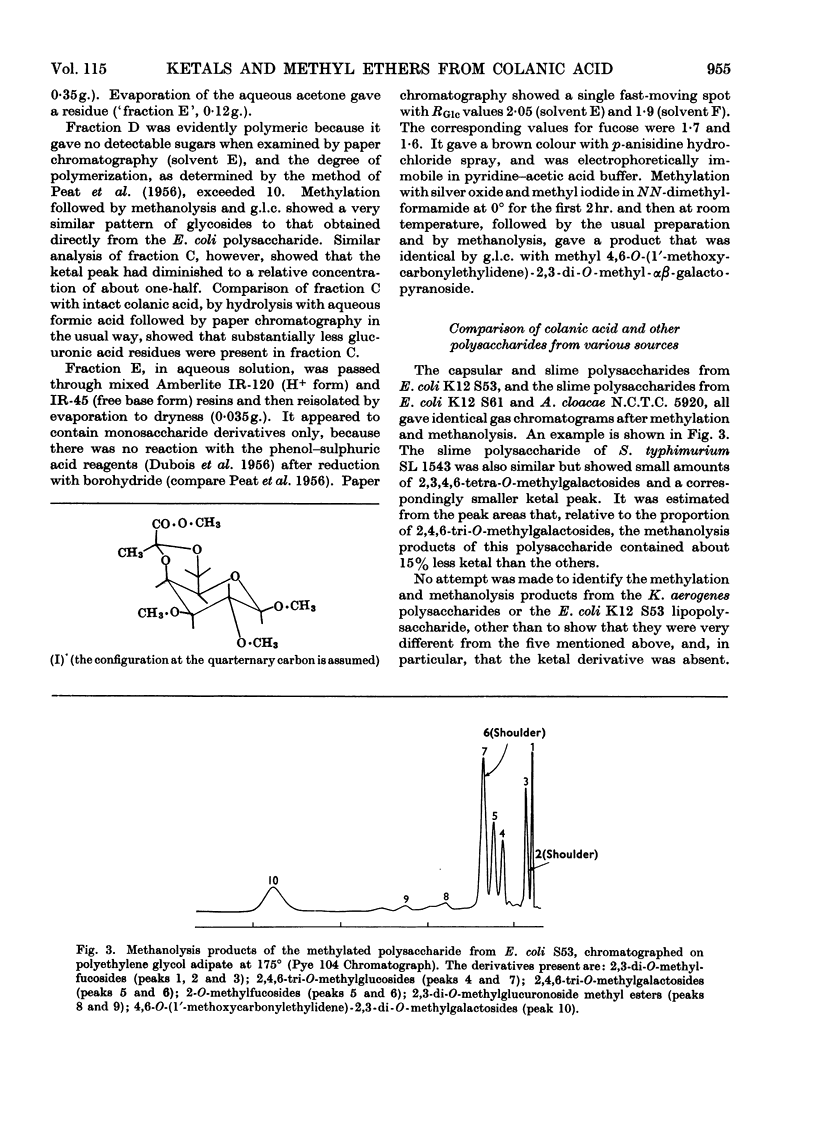
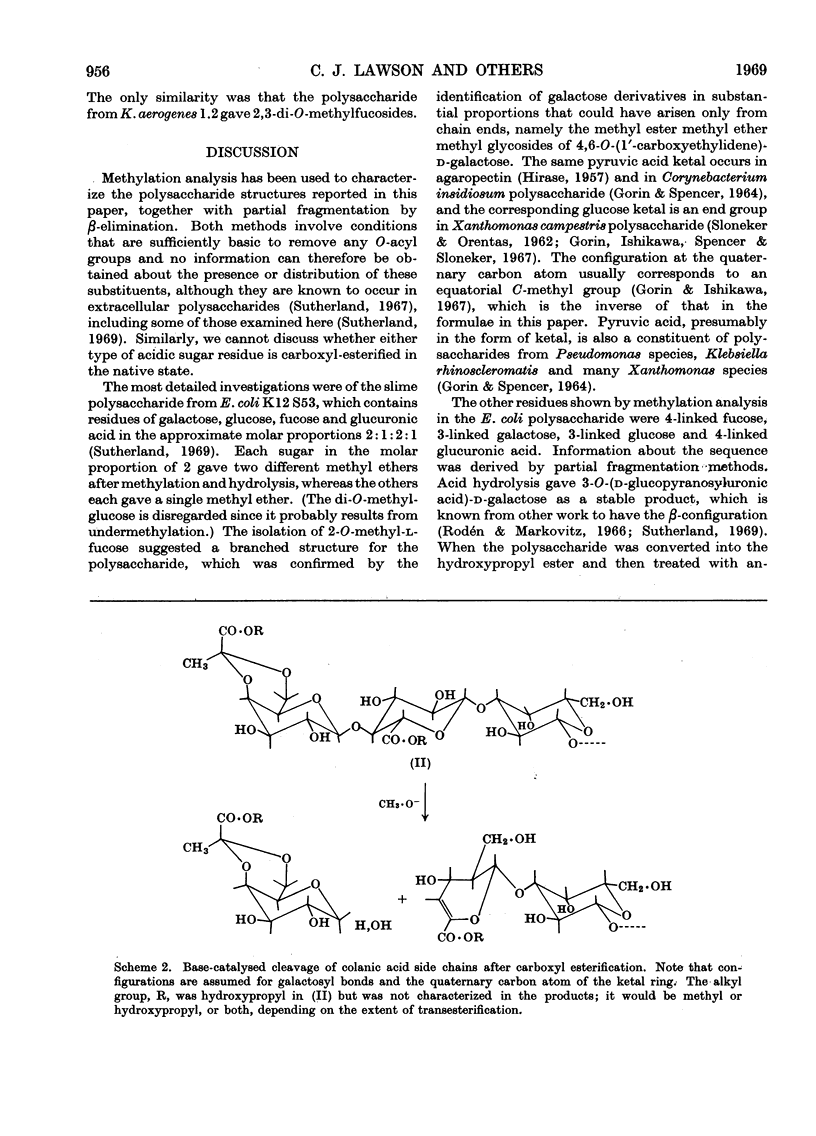
Abstract
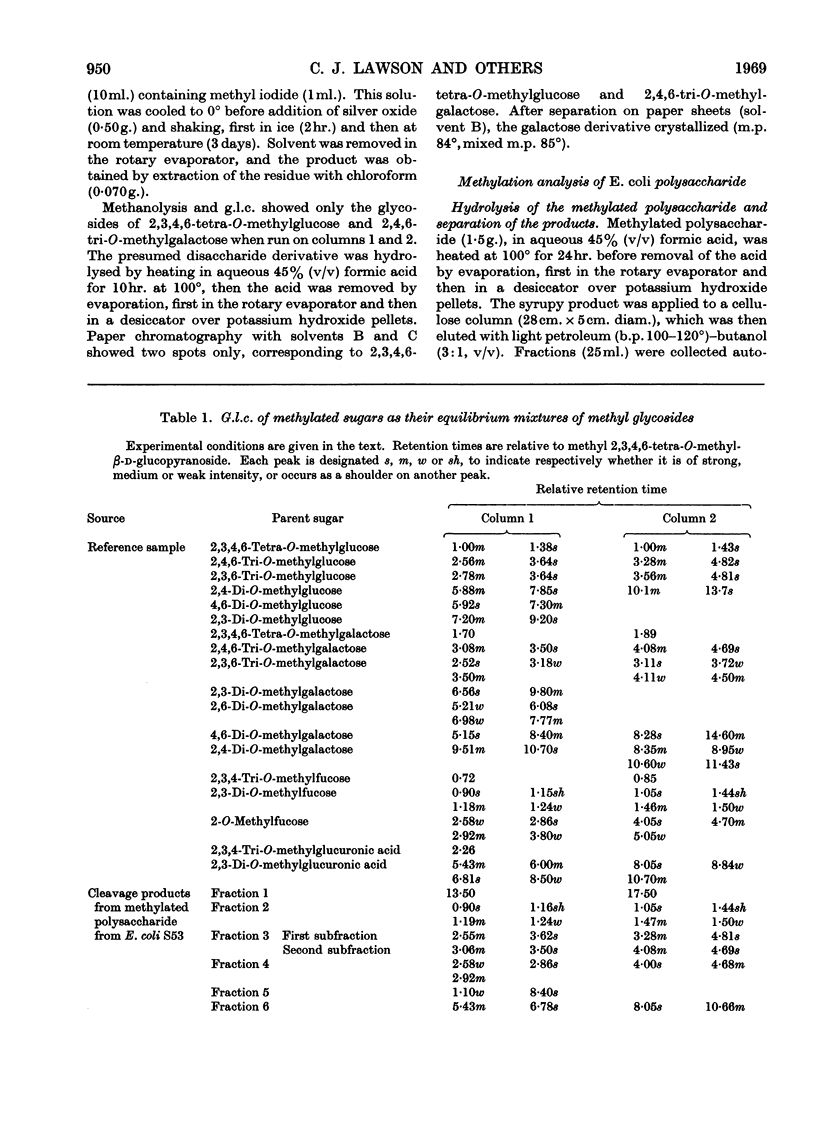
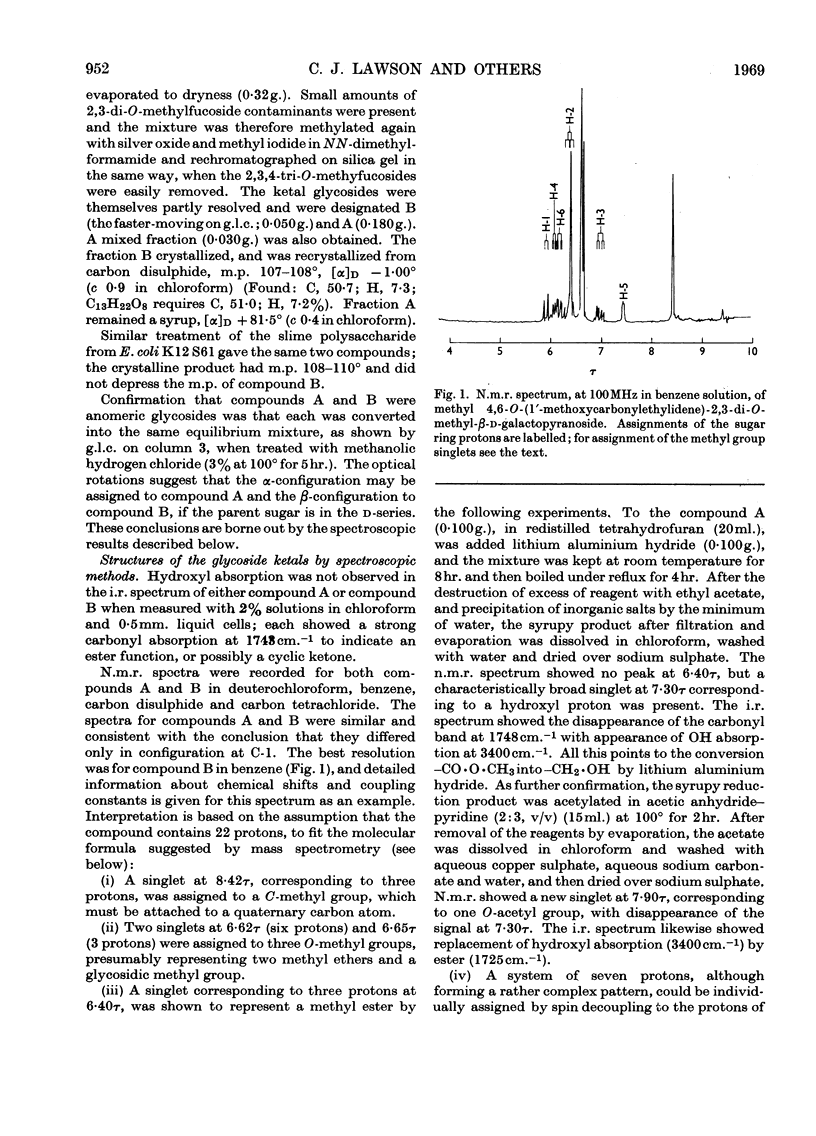
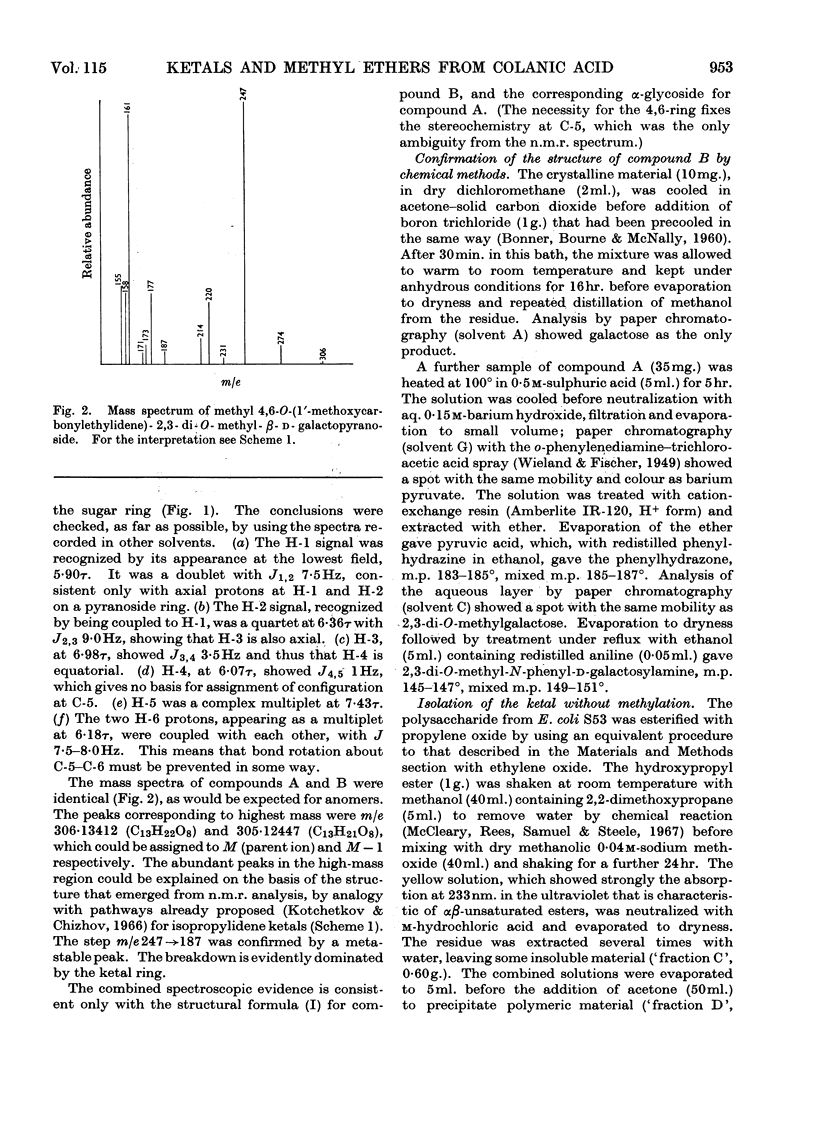
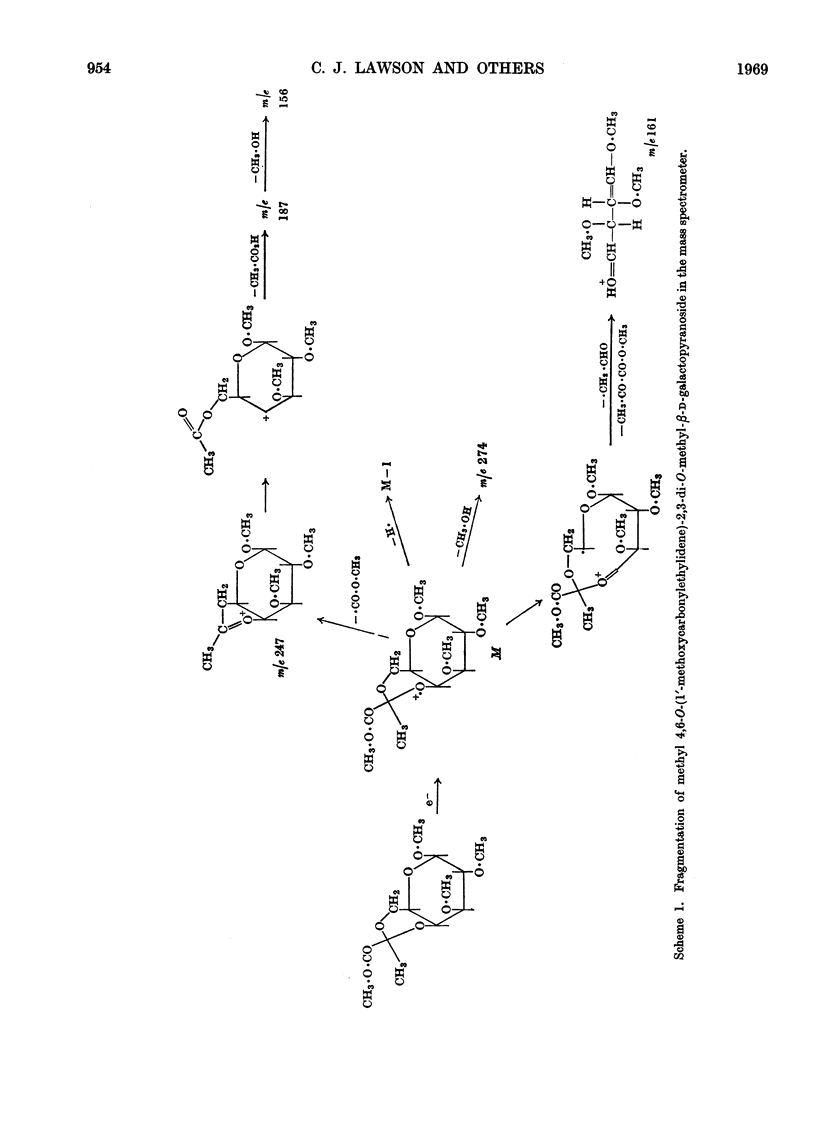
Essentially the same methanolysis products were obtained after methylation of the slime and capsular polysaccharides from Escherichia coli K12 (S53 and S53C sub-strains) and the slime polysaccharides from E. coli K12 (S61), Aerobacter cloacae N.C.T.C. 5290 and Salmonella typhimurium SL1543. These were the methyl glycosides of 2-O-methyl-l-fucose, 2,3-di-O-methyl-l-fucose, 2,3-di-O-methyl-d-glucuronic acid methyl ester, 2,4,6-tri-O-methyl-d-glucose, 2,4,6-tri-O-methyl-d-galactose and the pyruvic acid ketal, 4,6-O-(1′-methoxycarbonylethylidene)-2,3-O-methyl-d-galactose. All were identified as crystalline derivatives from an E. coli polysaccharide. The structure of the ketal was proved by proton-magnetic-resonance and mass spectrometry, and by cleavage to pyruvic acid and 2,3-di-O-methyl-d-galactose. All these polysaccharides are therefore regarded as variants on the same fundamental structure for which the name colanic acid is adopted. Although containing the same sugar residues, quite different methanolysis products were obtained after methylation of the extracellular polysaccharide from Klebsiella aerogenes (1.2 strain). The hydroxypropyl ester of E. coli polysaccharide, when treated with base under anhydrous conditions, underwent β-elimination at the uronate residues with release of a 4,6-O-(1′-alkoxycarbonylethylidene)-d-galactose. Together with the identification of 3-O-(d-glucopyranosyluronic acid)-d-galactose as a partial hydrolysis product, this establishes the nature of most, if not all, of the side chains as O-[4,6-O-(1′-carboxyethylidene)-d-galactopyranosyl]-(1→4)-O-(d-glucopyranosyluronic acid)-(1→3)-d-galactopyranosyl...
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Conrad H. E., Bamburg J. R., Epley J. D., Kindt T. J. The structure of the Aerobacter aerogenes A3(S1) polysaccharide. II. Sequence analysis and hydrolysis studies. Biochemistry. 1966 Sep;5(9):2808–2817. doi: 10.1021/bi00873a005. [DOI] [PubMed] [Google Scholar]
- DUDMAN W. F., WILKINSON J. F. The composition of the extracellular polysaccharides of Aerobacter-Klebsiella strains. Biochem J. 1956 Feb;62(2):289–295. doi: 10.1042/bj0620289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahan L. C., Sandford P. A., Conrad H. E. The structure of the serotype 2 capsular polysaccharide of Aerobacter aerogenes. Biochemistry. 1967 Sep;6(9):2755–2767. doi: 10.1021/bi00861a016. [DOI] [PubMed] [Google Scholar]
- KAUFFMANN F., LUEDERITZ O., STIERLIN H., WESTPHAL O. [On the immunochemistry of O antigens of Enterobacteriaceae. I. Analysis of the sugar component of Salmonella O antigens]. Zentralbl Bakteriol. 1960 May;178:442–458. [PubMed] [Google Scholar]
- Kochetkov N. K., Chizhov O. S. Mass spectrometry of carbohydrate derivatives. Adv Carbohydr Chem Biochem. 1966;21:39–93. doi: 10.1016/s0096-5332(08)60315-x. [DOI] [PubMed] [Google Scholar]
- Sandford P. A., Conrad H. E. The structure of the Aerobacter aerogenes A3(S1) polysaccharide. I. A reexamination using improved procedures for methylation analysis. Biochemistry. 1966 May;5(5):1508–1517. doi: 10.1021/bi00869a009. [DOI] [PubMed] [Google Scholar]
- Sutherland I. W. Phage-induced fucosidases hydrolysing the exopolysaccharide of Klebsiella arogenes type 54 [A3(S1)]. Biochem J. 1967 Jul;104(1):278–285. doi: 10.1042/bj1040278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland I. W. Structural studies on colanic acid, the common exopolysaccharide found in the enterobacteriaceae, by partial acid hydrolysis. Oligosaccharides from colanic acid. Biochem J. 1969 Dec;115(5):935–945. doi: 10.1042/bj1150935. [DOI] [PMC free article] [PubMed] [Google Scholar]


