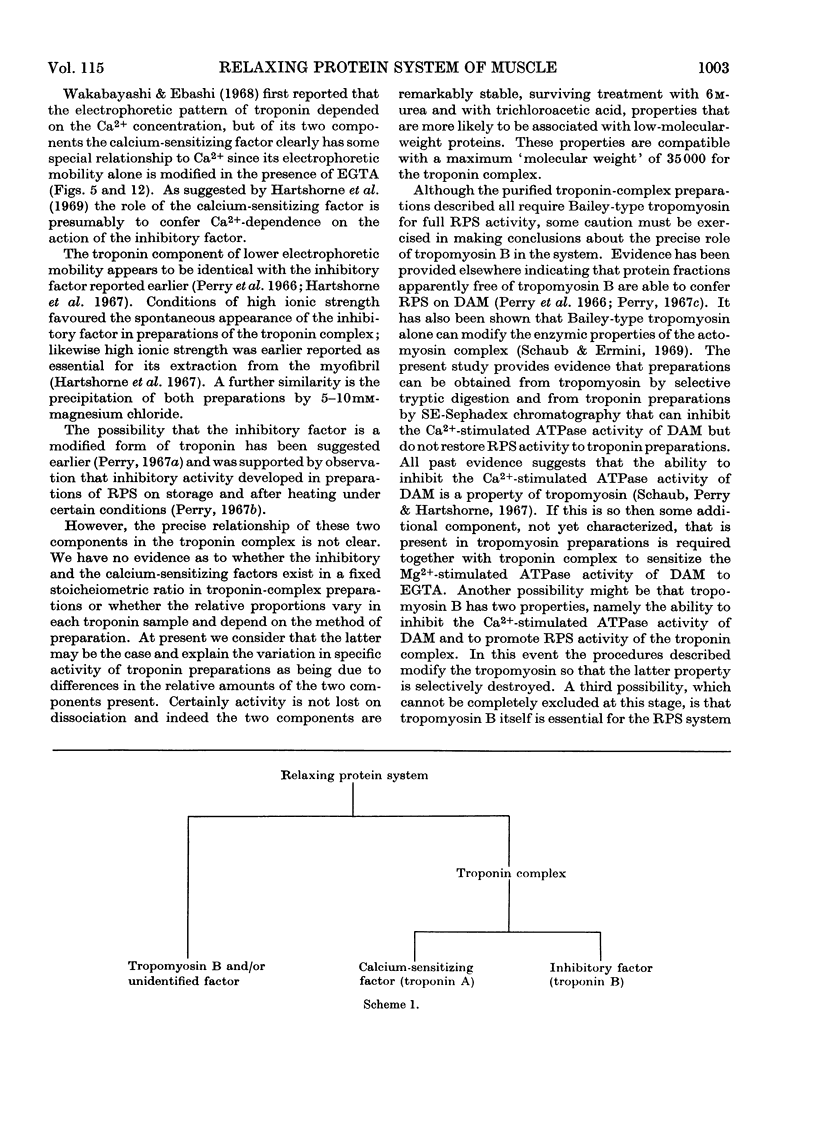
Abstract
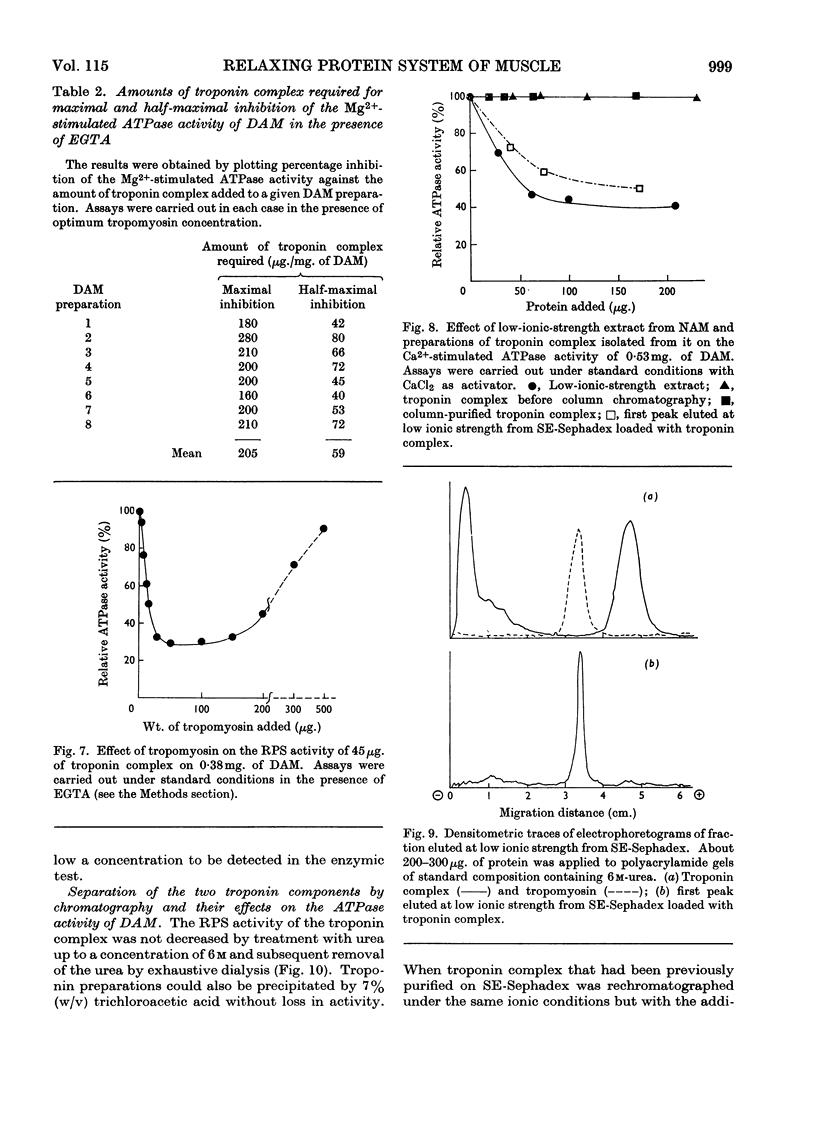
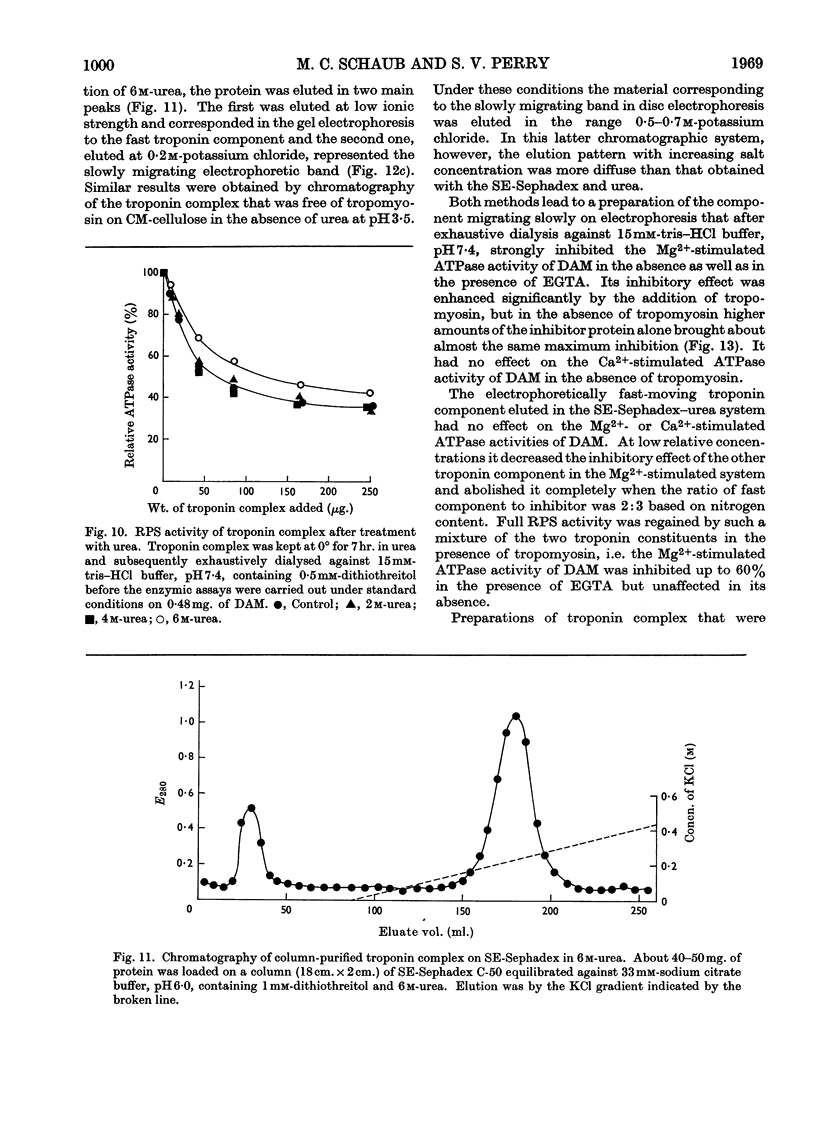
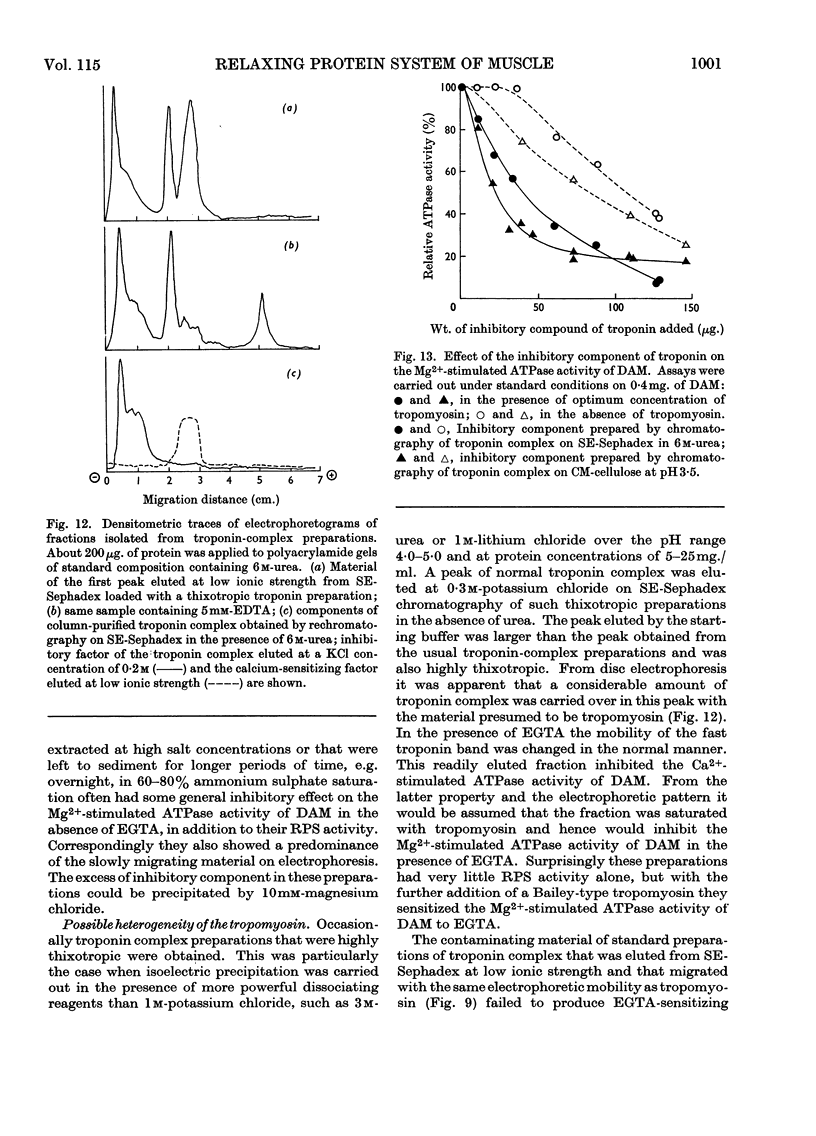
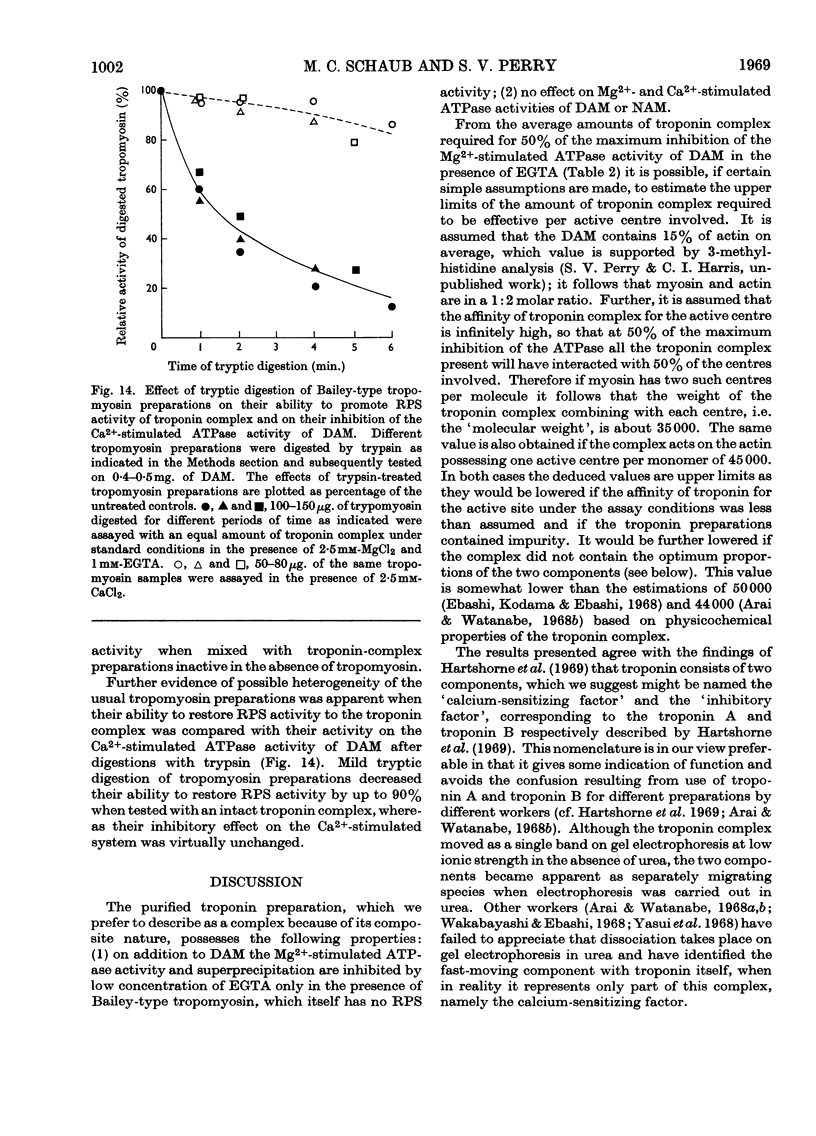
1. A method involving isoelectric precipitation and chromatography on SE-Sephadex (sulphoethyl-Sephadex) is described for the preparation of the troponin complex free of tropomyosin from low-ionic-strength extracts of natural actomyosin and myofibrils. 2. Purified troponin complex required tropomyosin to inhibit the Mg2+-stimulated adenosine triphosphatase activity and superprecipitation of desensitized actomyosin in the presence of ethanedioxybis(ethylamine)tetra-acetate. An upper limit of 35000 for the `molecular weight' of the troponin complex was derived from the amounts required to bring about 50% of the maximum inhibition of the Mg2+-stimulated adenosine triphosphatase activity of desensitized actomyosin of known concentration. 3. In the presence of dissociating reagents the troponin complex could be dissociated into inhibitory and Ca2+-sensitizing factors, which could be isolated separately on SE-Sephadex. The inhibitory factor inhibited the Mg2+-stimulated adenosine triphosphatase activity and superprecipitation of desensitized actomyosin independently of the concentration of free Ca2+ in the medium. 4. The Ca2+-sensitizing factor changed its electrophoretic mobility on polyacrylamide gel in the presence of ethanedioxybis(ethylamine)tetra-acetate. It formed a complex with the inhibitory factor at low ionic strength and the original biological activity of the troponin complex could be restored on mixing the inhibitory factor with the Ca2+-sensitizing factor in the ratio of about 3:2. 5. Evidence is presented indicating that the ability of tropomyosin preparations to restore relaxing-protein-system activity to the troponin complex and their inhibitory effect on the Ca2+-stimulated adenosine triphosphatase activity of desensitized actomyosin are two properties of different stability to preparative procedures and tryptic digestion. This suggests that the relaxing protein system of muscle may contain another as yet uncharacterized component.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai K. I., Watanabe S. A study of troponin, a myofibrillar protein from rabbit skeletal muscle. J Biol Chem. 1968 Nov 10;243(21):5670–5678. [PubMed] [Google Scholar]
- Bailey K. Tropomyosin: a new asymmetric protein component of the muscle fibril. Biochem J. 1948;43(2):271–279. doi: 10.1042/bj0430271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennick A. Detection of persulfate in acrylamide gels. Anal Biochem. 1968 Dec;26(3):453–456. doi: 10.1016/0003-2697(68)90209-1. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- EBASHI S., EBASHI F. A NEW PROTEIN COMPONENT PARTICIPATING IN THE SUPERPRECIPITATION OF MYOSIN B. J Biochem. 1964 Jun;55:604–613. doi: 10.1093/oxfordjournals.jbchem.a127933. [DOI] [PubMed] [Google Scholar]
- EBASHI S. THIRD COMPONENT PARTICIPATING IN THE SUPERPRECIPITATION OF 'NATURAL ACTOMYOSIN'. Nature. 1963 Dec 7;200:1010–1010. doi: 10.1038/2001010a0. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Kodama A., Ebashi F. Troponin. I. Preparation and physiological function. J Biochem. 1968 Oct;64(4):465–477. doi: 10.1093/oxfordjournals.jbchem.a128918. [DOI] [PubMed] [Google Scholar]
- Hartshorne D. J., Mueller H. Fractionation of troponin into two distinct proteins. Biochem Biophys Res Commun. 1968 Jun 10;31(5):647–653. doi: 10.1016/0006-291x(68)90610-4. [DOI] [PubMed] [Google Scholar]
- Hartshorne D. J., Mueller H. The preparation of tropomyosin and troponin from natural actomyosin. Biochim Biophys Acta. 1969 Mar;175(2):301–319. doi: 10.1016/0005-2795(69)90008-7. [DOI] [PubMed] [Google Scholar]
- Hartshorne D. J., Perry S. V., Schaub M. C. A protein factor inhibiting the magnesium-activated adenosine triphosphatase of desensitized actomyosin. Biochem J. 1967 Sep;104(3):907–913. doi: 10.1042/bj1040907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorne D. J., Theiner M., Mueller H. Studies on troponin. Biochim Biophys Acta. 1969 Mar;175(2):320–330. doi: 10.1016/0005-2795(69)90009-9. [DOI] [PubMed] [Google Scholar]
- PERRY S. V., CORSI A. Extraction of proteins other than myosin from the isolated rabbit myofibril. Biochem J. 1958 Jan;68(1):5–12. doi: 10.1042/bj0680005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRY S. V., ZYDOWO M. The nature of the extra protein fraction from myofibrils of striated muscle. Biochem J. 1959 Feb;71(2):220–228. doi: 10.1042/bj0710220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons A. L., Parsons J. L., Blanshard J. M., Lawrie R. A. Electrophoretic differentiaion f myofibrillar proteins in the pig. Biochem J. 1969 May;112(5):673–678. doi: 10.1042/bj1120673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry S. V., Cotterill J., Hayter D. The adenosine-triphosphatase activity of dissociated acto-heavy-meromyosin. Biochem J. 1966 Aug;100(2):289–294. doi: 10.1042/bj1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWARTZ A., BACHELARD H. S., McIL WAIN H. The sodium-stimulated adenosine-triphosphatase activity and other properties of cerebral microsomal fractions and subfractions. Biochem J. 1962 Sep;84:626–637. doi: 10.1042/bj0840626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub M. C., Ermini M. Effect of bivalent cations on the adenosine triphosphatase of actomyosin and its modification by tropomyosin and troponin. Biochem J. 1969 Mar;111(5):777–783. doi: 10.1042/bj1110777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub M. C., Hartshorne D. J., Perry S. V. Effect of tropomyosin on the calcium-activated adenosine triphosphatase of actomyosin. Nature. 1967 Aug 5;215(5101):635–636. doi: 10.1038/215635a0. [DOI] [PubMed] [Google Scholar]
- Schaub M. C., Hartshorne D. J., Perry S. V. The adeonosine-triphosphatase activity of desensitized actomyosin. Biochem J. 1967 Jul;104(1):263–269. doi: 10.1042/bj1040263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub M. C., Perry S. V., Hartshorne D. J. The effect of tropomyosin on the adenosine triphosphatase activity of desensitized actomyosin. Biochem J. 1967 Dec;105(3):1235–1243. doi: 10.1042/bj1051235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub M. C., Strauch L. Purification of collagenase. IV. Separation and identification of clostridial collagenases by disc electrophoresis on polyacrylamide gel. Hoppe Seylers Z Physiol Chem. 1968 Jun;349(6):809–815. doi: 10.1515/bchm2.1968.349.1.809. [DOI] [PubMed] [Google Scholar]
- WEBER A., HERZ R. The binding of calcium to actomyosin systems in relation to their biological activity. J Biol Chem. 1963 Feb;238:599–605. [PubMed] [Google Scholar]
- Wakabayashi T., Ebashi S. Reversible change in physical state of troponin induced by calcium ion. J Biochem. 1968 Nov;64(5):731–732. doi: 10.1093/oxfordjournals.jbchem.a128955. [DOI] [PubMed] [Google Scholar]
- Woods E. F. Molecular weight and subunit structure of tropomyosin B. J Biol Chem. 1967 Jun 25;242(12):2859–2871. [PubMed] [Google Scholar]
- Yasui B., Fuchs F., Briggs F. N. The role of the sulfhydryl groups of tropomyosin and troponin in the calcium control of actomyosin contractility. J Biol Chem. 1968 Feb 25;243(4):735–742. [PubMed] [Google Scholar]


