Abstract
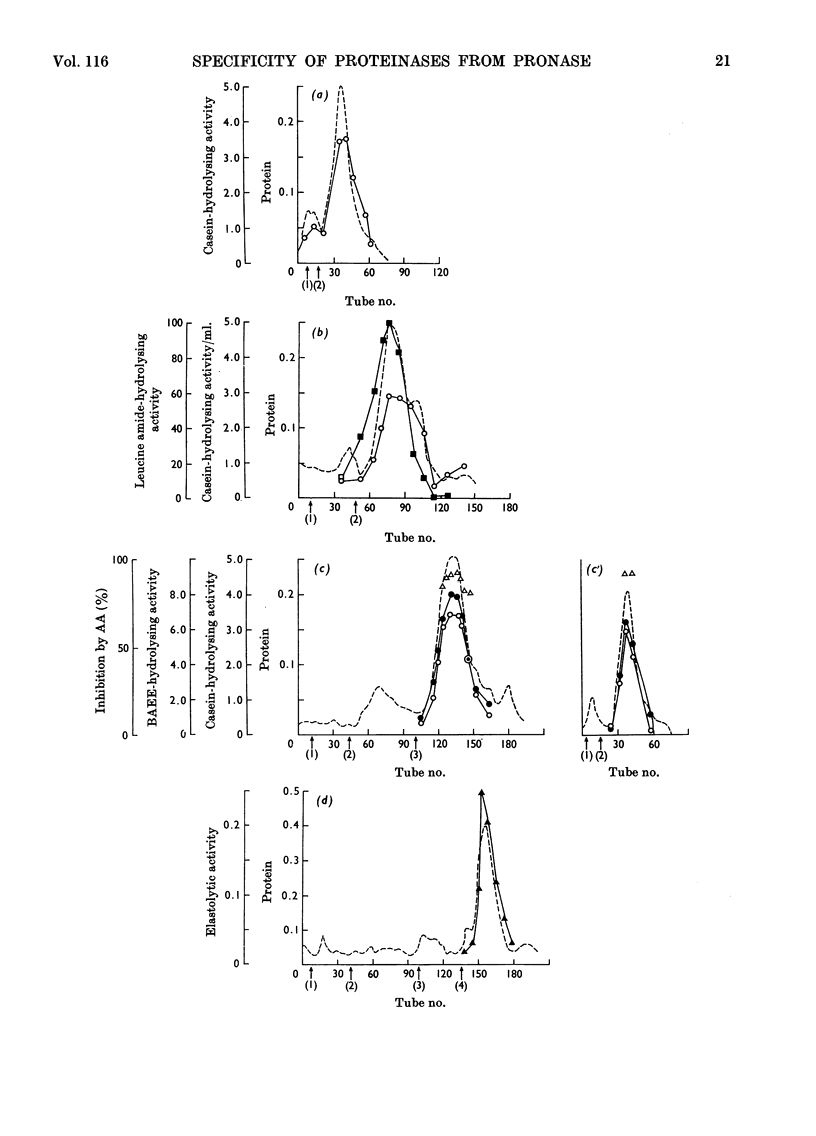
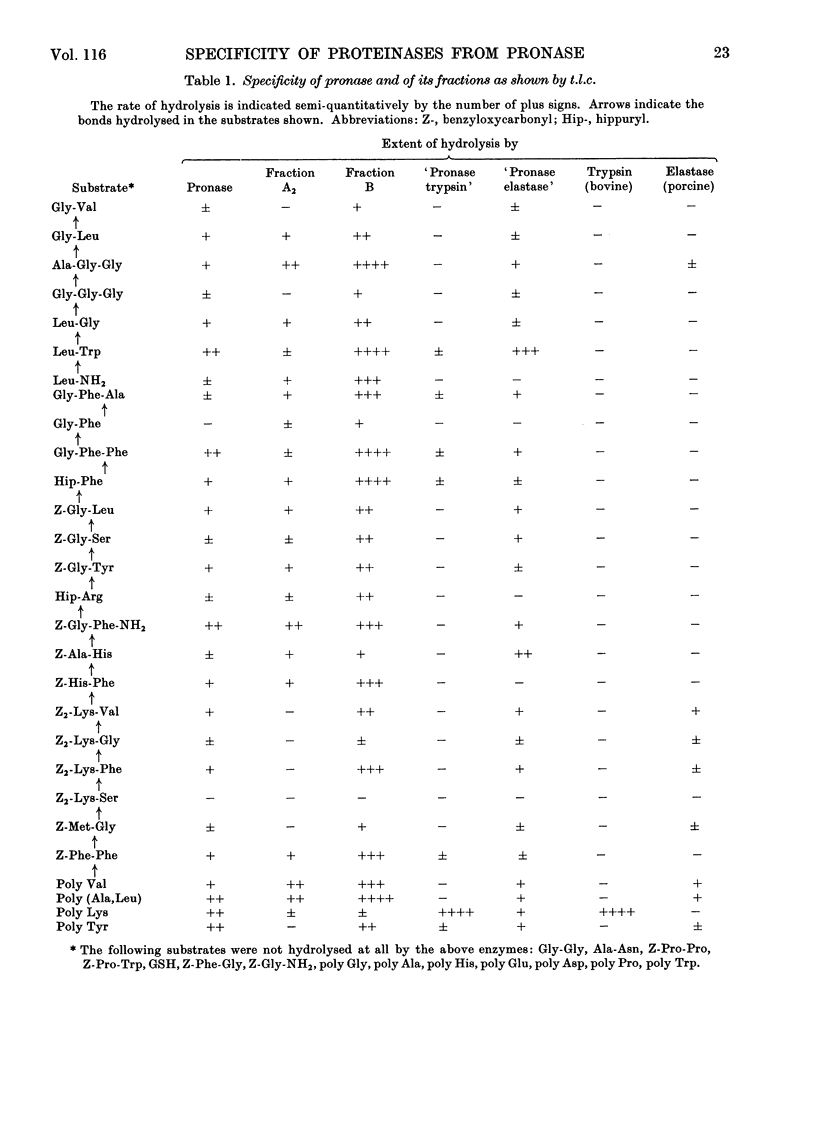
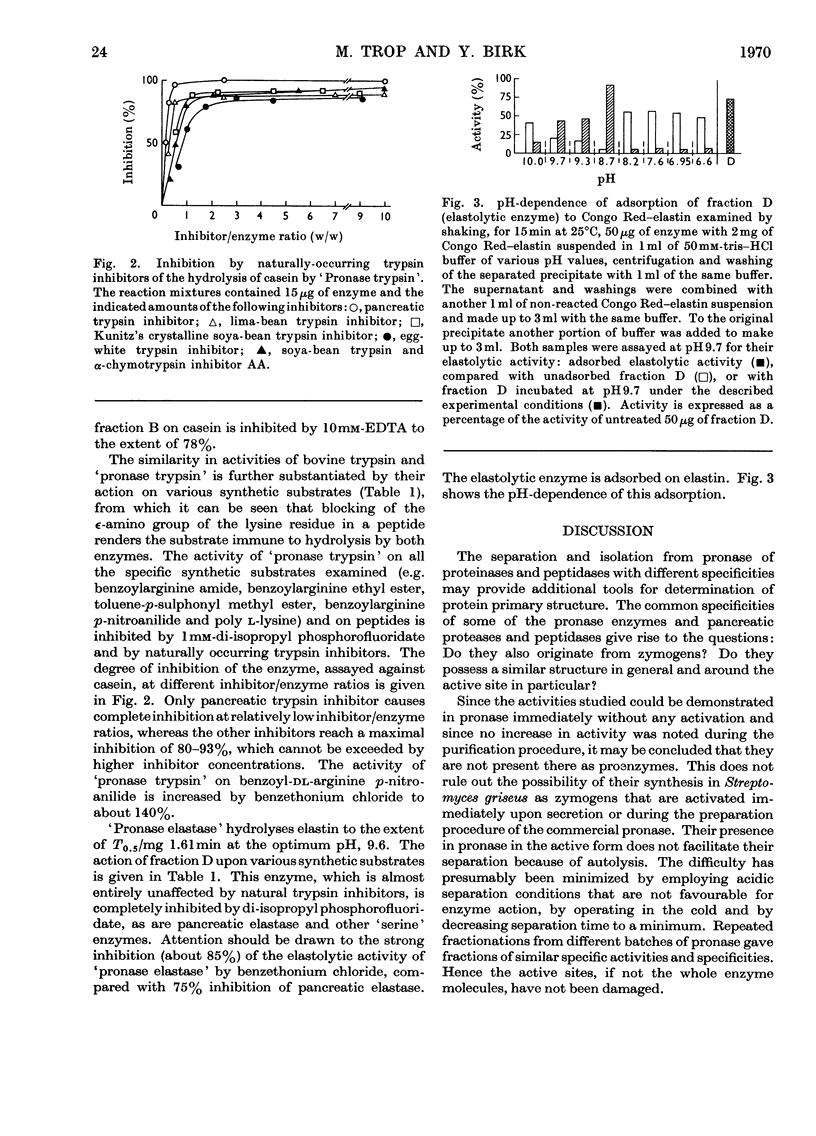
Purification of pronase by ion-exchange chromatography gave four proteolytically active fractions. Fraction A2 contained an endopeptidase that attacks poly l-valine. Fraction B contained an endopeptidase, an aminopeptidase and carboxypeptidases. The activities against hippuryl-l-arginine and hippuryl-l-phenylalanine could be inhibited to a considerable extent by di-isopropyl phosphorofluoridate and by EDTA. Fraction C contained an endopeptidase resembling bovine trypsin. The pure enzyme was completely inactivated by di-isopropyl phosphorofluoridate and pancreatic trypsin inhibitor and to about 90% by other naturally occurring trypsin inhibitors. Fraction D contained an apparently homogeneous endopeptidase, inhibited by diisopropyl phosphorofluoridate, that adsorbed to and hydrolysed elastin. The activity of all these fractions was tested qualitatively against a wide range of small peptides and synthetic substrates.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIRK Y., GERTLER A., KHALEF S. A pure trypsin inhibitor from soya beans. Biochem J. 1963 May;87:281–284. doi: 10.1042/bj0870281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERLANGER B. F., KOKOWSKY N., COHEN W. The preparation and properties of two new chromogenic substrates of trypsin. Arch Biochem Biophys. 1961 Nov;95:271–278. doi: 10.1016/0003-9861(61)90145-x. [DOI] [PubMed] [Google Scholar]
- Gertler A., Hofmann T. The involvement of the amino-terminal amino acid in the activity of pancreatic proteases. I. The effects of nitrous acid on elastase. J Biol Chem. 1967 May 25;242(10):2522–2527. [PubMed] [Google Scholar]
- HIRAMATSU A., OUCHI T. ON THE PROTEOLYTIC ENZYMES FROM THE COMMERCIAL PROTEASE PREPARATION OF STREPTOMYCES GRISEUS (PRONASE P). J Biochem. 1963 Nov;54:462–464. doi: 10.1093/oxfordjournals.jbchem.a127815. [DOI] [PubMed] [Google Scholar]
- HUMMEL B. C. A modified spectrophotometric determination of chymotrypsin, trypsin, and thrombin. Can J Biochem Physiol. 1959 Dec;37:1393–1399. [PubMed] [Google Scholar]
- MITZ M. A., SCHLUETER R. J. Direct spectrophotometric measurement of the peptide bond; application to the determination of acylase I. Biochim Biophys Acta. 1958 Jan;27(1):168–172. doi: 10.1016/0006-3002(58)90305-6. [DOI] [PubMed] [Google Scholar]
- Narahashi Y., Shibuya K., Yanagita M. Studies on proteolytic enzymes (pronase) of Streptomyces griseus K-1. II. Separation of exo- and endopeptidases of pronase. J Biochem. 1968 Oct;64(4):427–437. doi: 10.1093/oxfordjournals.jbchem.a128914. [DOI] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- Ryan C. A. Chymotrypsin inhibitor I from potatoes: reactivity with mammalian, plant, bacterial, and fungal proteinases. Biochemistry. 1966 May;5(5):1592–1596. doi: 10.1021/bi00869a020. [DOI] [PubMed] [Google Scholar]
- SCHWERT G. W., TAKENAKA Y. A spectrophotometric determination of trypsin and chymotrypsin. Biochim Biophys Acta. 1955 Apr;16(4):570–575. doi: 10.1016/0006-3002(55)90280-8. [DOI] [PubMed] [Google Scholar]
- Trop M., Birk Y. The trypsin-like enzyme from Streptomyces griseus (pronase). Biochem J. 1968 Sep;109(3):475–476. doi: 10.1042/bj1090475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wählby S., Engström L. Studies on Streptomyces griseus protease. II. The amino acid sequence around the reactive serine residue of DFP-sensitive components with esterase activity. Biochim Biophys Acta. 1968 Feb 5;151(2):402–408. doi: 10.1016/0005-2744(68)90107-1. [DOI] [PubMed] [Google Scholar]
- Wählby S. Studies on Streptomyces griseus protease. I. Separation of DFP-reacting enzymes and purification of one of the enzymes. Biochim Biophys Acta. 1968 Feb 5;151(2):394–401. doi: 10.1016/0005-2744(68)90106-x. [DOI] [PubMed] [Google Scholar]
- Wählby S., Zetterqvist O., Engström L. Reaction of two enzyme fractions from Streptomyces griseus protease with diisopropylphosphoro-fluoridate. Acta Chem Scand. 1965;19(5):1247–1248. doi: 10.3891/acta.chem.scand.19-1247. [DOI] [PubMed] [Google Scholar]


