Abstract
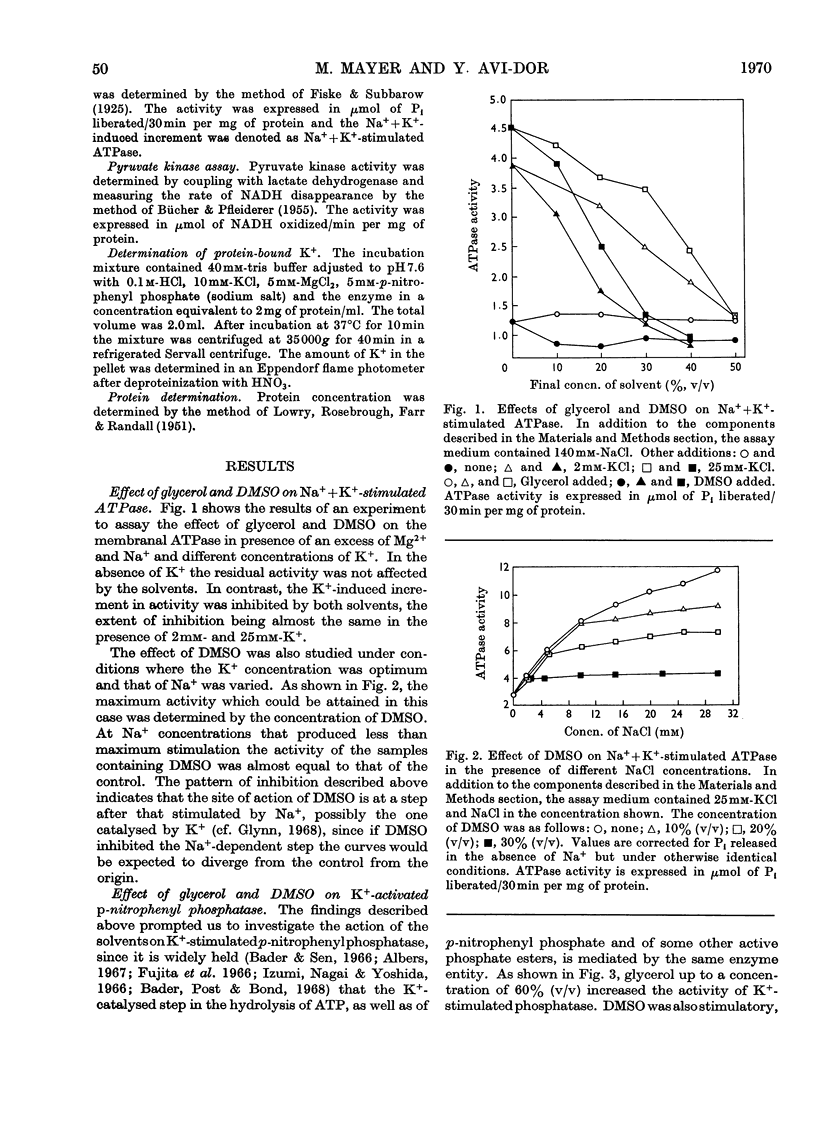
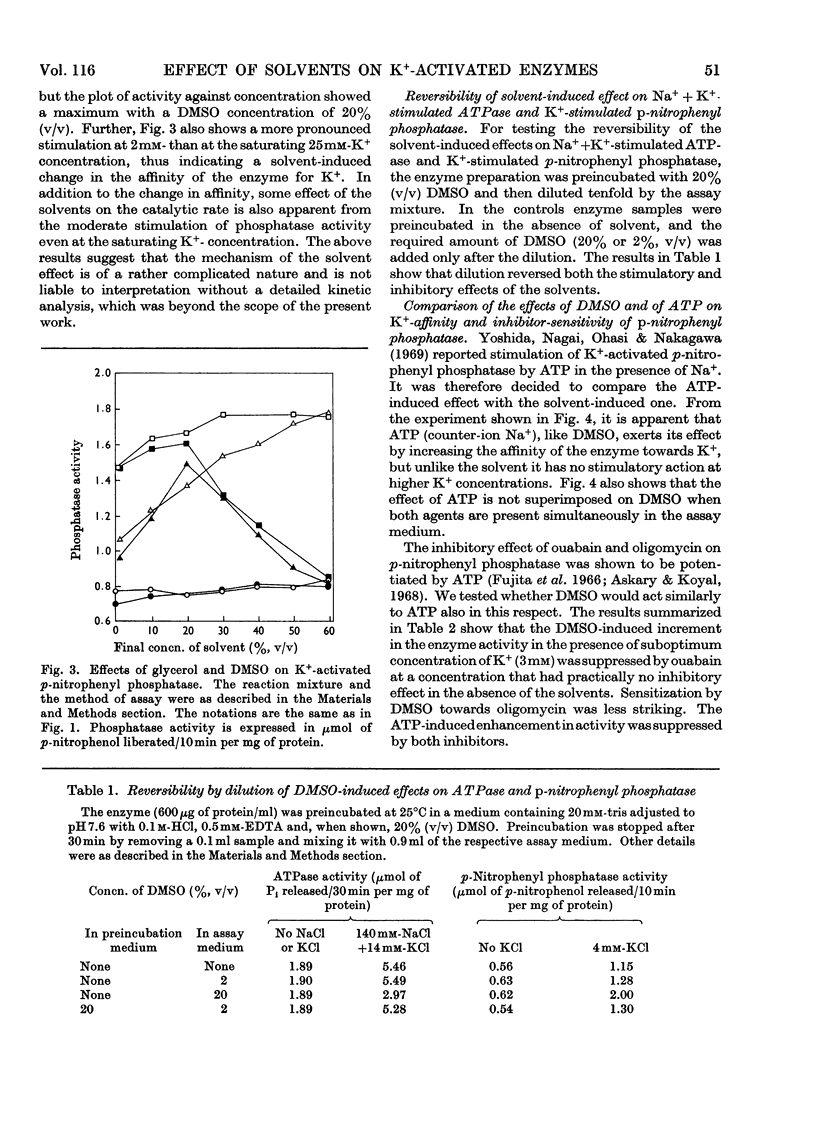
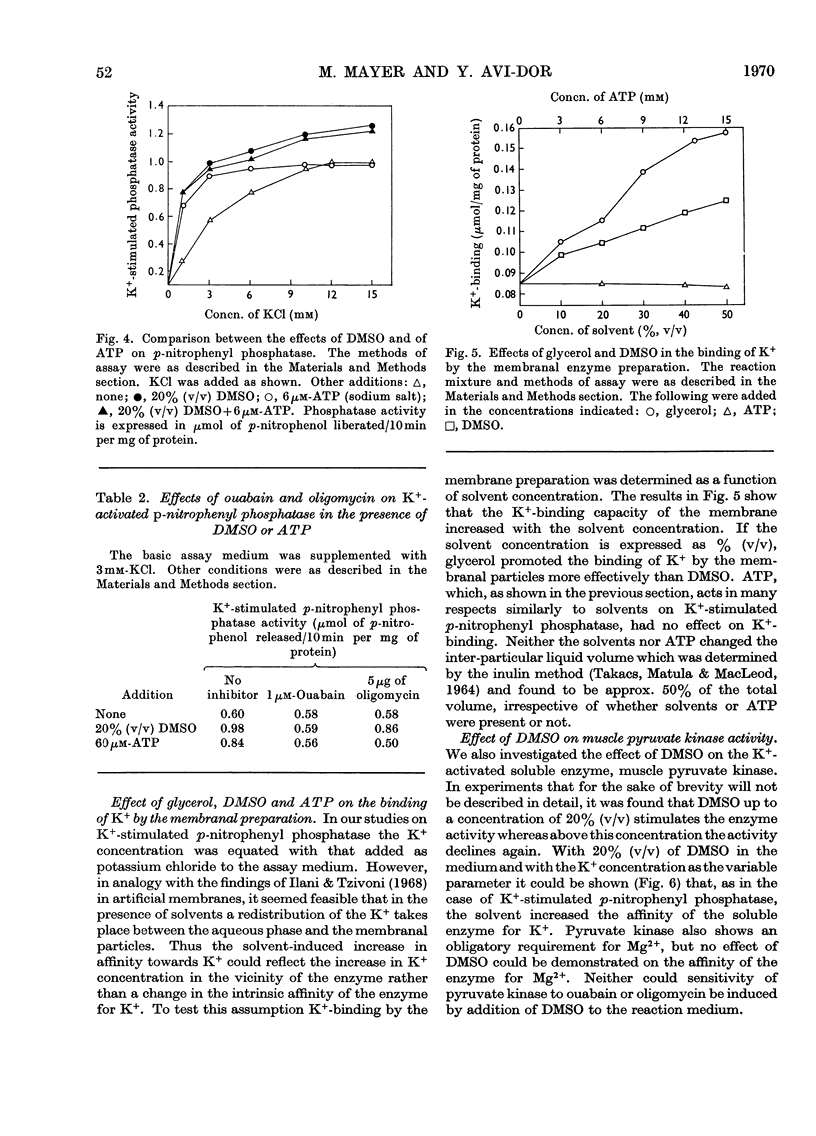
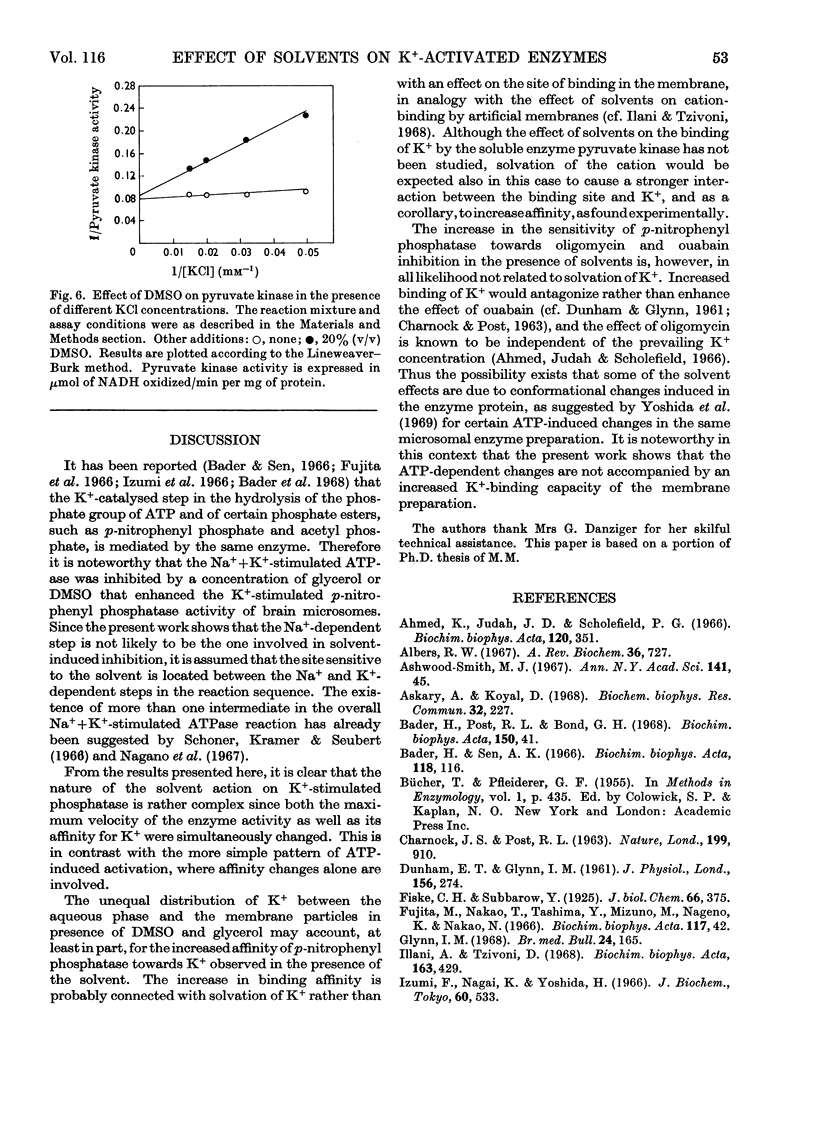
The effects of dimethyl sulphoxide and glycerol on ox brain microsomal Na++K+-stimulated adenosine triphosphatase (EC 3.6.1.3), K+-stimulated p-nitrophenyl phosphatase and K+-dependent muscle pyruvate kinase (EC 2.7.1.40) were studied. Dimethyl sulphoxide at concentrations below 20% (v/v) was found to stimulate the p-nitrophenyl phosphatase and pyruvate kinase by increasing their affinity for K+ but to inhibit the Na++K+-stimulated adenosine triphosphatase. The latter enzyme activity was also inhibited by glycerol, which like dimethyl sulphoxide, stimulated the K+-activated p-nitrophenyl phosphatase at a wide range of concentrations. The solvent effects were promptly reversed by dilution. Similarity was found between glycerol and dimethyl sulphoxide, on one hand, and ATP, on the other, in their stimulatory effect and their ability to increase the ouabain- and oligomycin-sensitivity of the K+-stimulated p-nitrophenyl phosphatase. However, only the solvents, not the ATP, increased the binding of K+ by the microsomes. From the above findings it is suggested that solvents may act on K+-dependent enzymes by altering the state of solvation of the activating cation as well as by changing the enzyme structure.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed K., Judah J. D., Scholefield P. G. Interaction of sodium and potassium with a cation-dependent adenosine triphosphatase system from rat brain. Biochim Biophys Acta. 1966 Jul 13;120(3):351–360. doi: 10.1016/0926-6585(66)90302-5. [DOI] [PubMed] [Google Scholar]
- Ashwood-Smith M. J. Radioprotective and cryoprotective properties of dimethyl sulfoxide in cellular systems. Ann N Y Acad Sci. 1967 Mar 15;141(1):45–62. doi: 10.1111/j.1749-6632.1967.tb34865.x. [DOI] [PubMed] [Google Scholar]
- Askari A., Koyal D. Different oligomycin sensitivities of the Na++K+-activated adenosinetriphosphatase and its partial reactions. Biochem Biophys Res Commun. 1968 Jul 26;32(2):227–232. doi: 10.1016/0006-291x(68)90373-2. [DOI] [PubMed] [Google Scholar]
- Bader H., Post R. L., Bond G. H. Comparison of sources of a phosphorylated intermediate in transport ATPase. Biochim Biophys Acta. 1968 Jan 3;150(1):41–46. doi: 10.1016/0005-2736(68)90006-0. [DOI] [PubMed] [Google Scholar]
- Bader H., Sen A. K. (K+)-dependent acyl phosphatase as part of the (na+ + K+)-dependent ATPase of cell membranes. Biochim Biophys Acta. 1966 Apr 12;118(1):116–123. doi: 10.1016/s0926-6593(66)80150-9. [DOI] [PubMed] [Google Scholar]
- DUNHAM E. T., GLYNN I. M. Adenosinetriphosphatase activity and the active movements of alkali metal ions. J Physiol. 1961 Apr;156:274–293. doi: 10.1113/jphysiol.1961.sp006675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M., Nakao T., Tashima Y., Mizuno N., Nagano K., Nakao M. Potassium-ion stimulated p-nitrophenylphosphatase activity occurring in a highly specific adenosine triphosphatase preparation from rabbit brain. Biochim Biophys Acta. 1966 Mar 28;117(1):42–53. doi: 10.1016/0304-4165(66)90150-4. [DOI] [PubMed] [Google Scholar]
- Glynn I. M. Membrane adenosine triphosphatase and cation transport. Br Med Bull. 1968 May;24(2):165–169. doi: 10.1093/oxfordjournals.bmb.a070620. [DOI] [PubMed] [Google Scholar]
- Ilani A., Tzivoni D. Hydrogen ions in hydrophobic membranes. Biochim Biophys Acta. 1968 Dec 10;163(4):429–438. doi: 10.1016/0005-2736(68)90072-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mnagano K., Mizuno N., Fujita M., Tashima Y., Nakao T., Nakao M. On the possible role of the phosphorylated intermediate in the reaction mechanism of (Na+-K+)-ATPase. Biochim Biophys Acta. 1967 Jul 5;143(1):239–248. doi: 10.1016/0005-2728(67)90124-7. [DOI] [PubMed] [Google Scholar]
- Nakao T., Tashima Y., Nagano K., Nakao M. Highly specific sodium-potassium-activated adenosine triphosphatase from various tissues of rabbit. Biochem Biophys Res Commun. 1965 Jun 9;19(6):755–758. doi: 10.1016/0006-291x(65)90323-2. [DOI] [PubMed] [Google Scholar]
- Schoner W., Kramer R., Seubert W. On the role of phosphorylated intermediates in sodium and potassium stimulated ATP-hydrolysis. Biochem Biophys Res Commun. 1966 May 25;23(4):403–408. doi: 10.1016/0006-291x(66)90741-8. [DOI] [PubMed] [Google Scholar]
- TAKACS F. P., MATULA T. I., MACLEOD R. A. NUTRITION AND METABOLISM OF MARINE BACTERIA. XIII. INTRACELLULAR CONCENTRATIONS OF SODIUM AND POTASSIUM IONS IN A MARINE PSEUDOMONAD. J Bacteriol. 1964 Mar;87:510–518. doi: 10.1128/jb.87.3.510-518.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Nagai K., Ohashi T., Nakagawa Y. K+ minus dependent phosphatase activity observed in the presence of both adenosine triphosphate and Na+. Biochim Biophys Acta. 1969 Jan 7;171(1):178–185. doi: 10.1016/0005-2744(69)90117-x. [DOI] [PubMed] [Google Scholar]


