Abstract
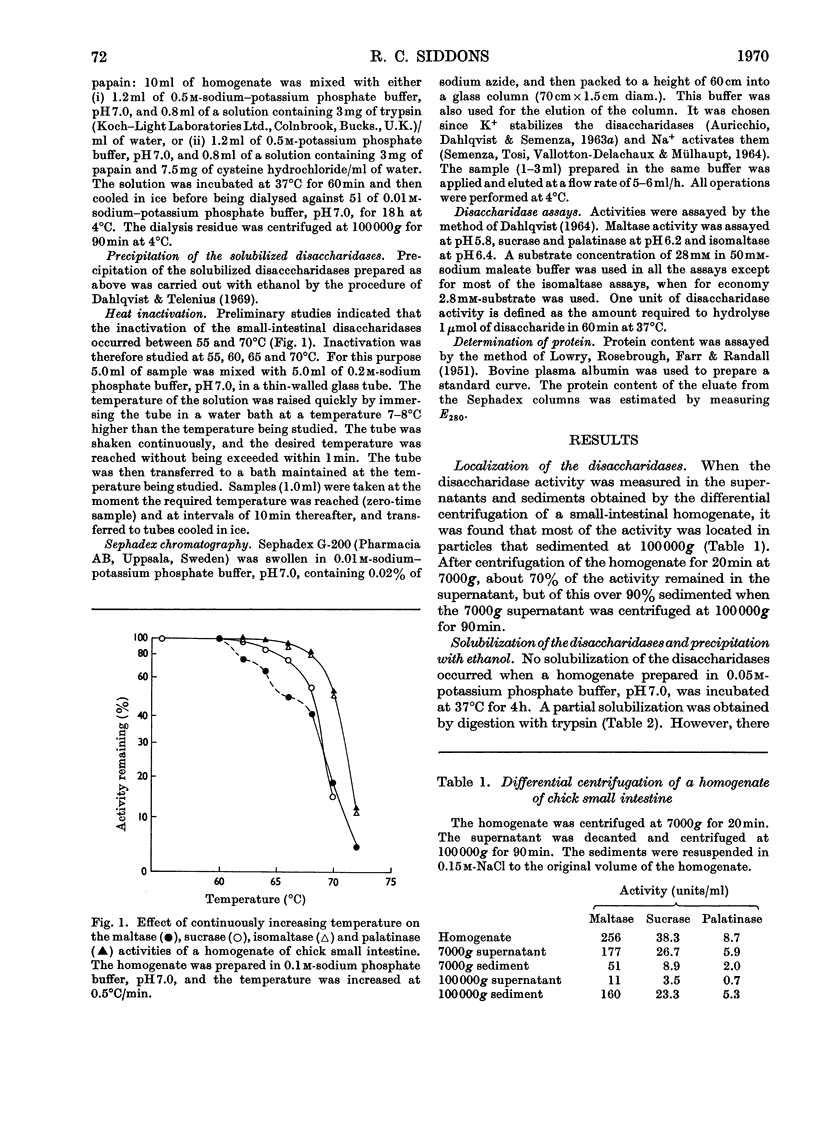
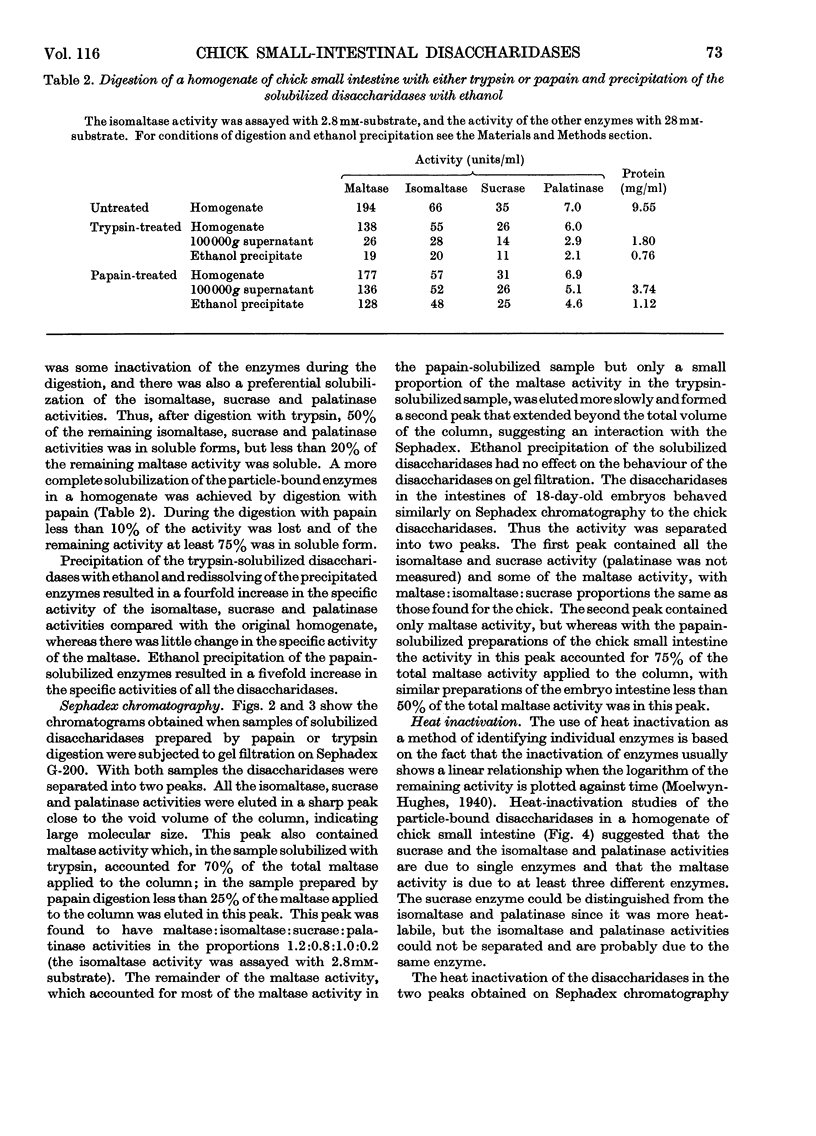
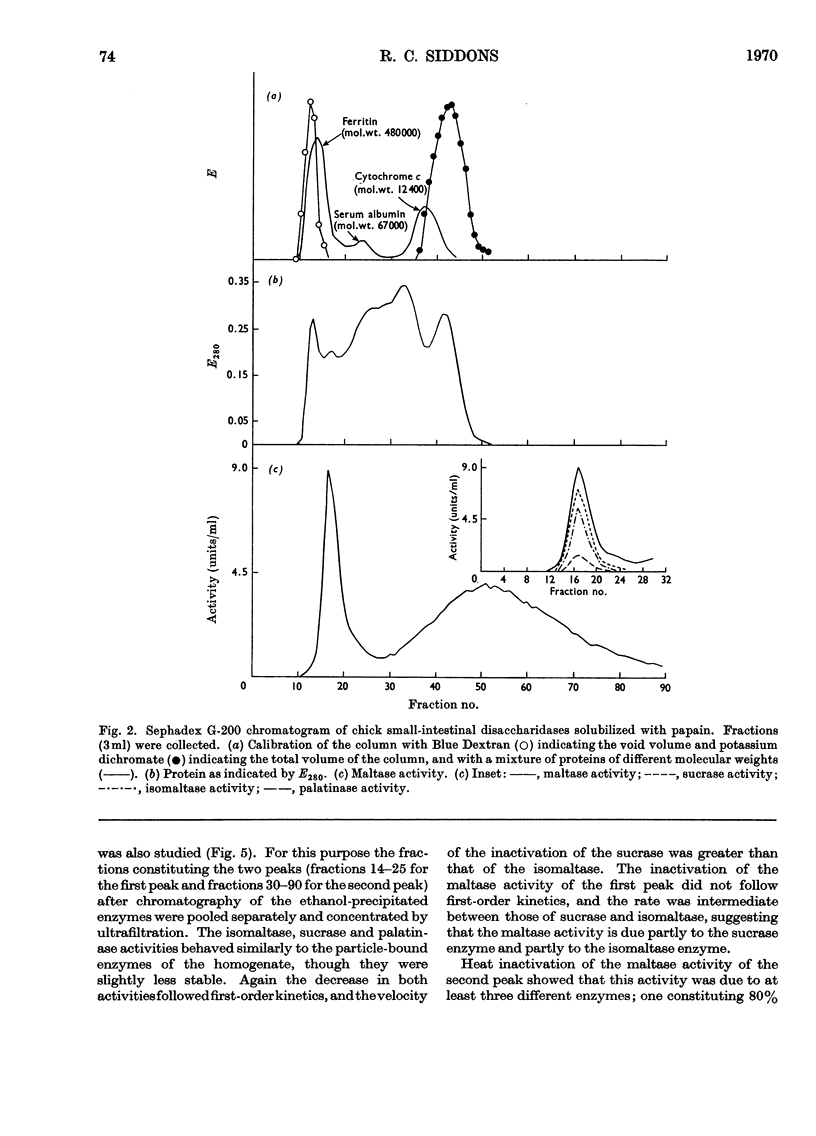
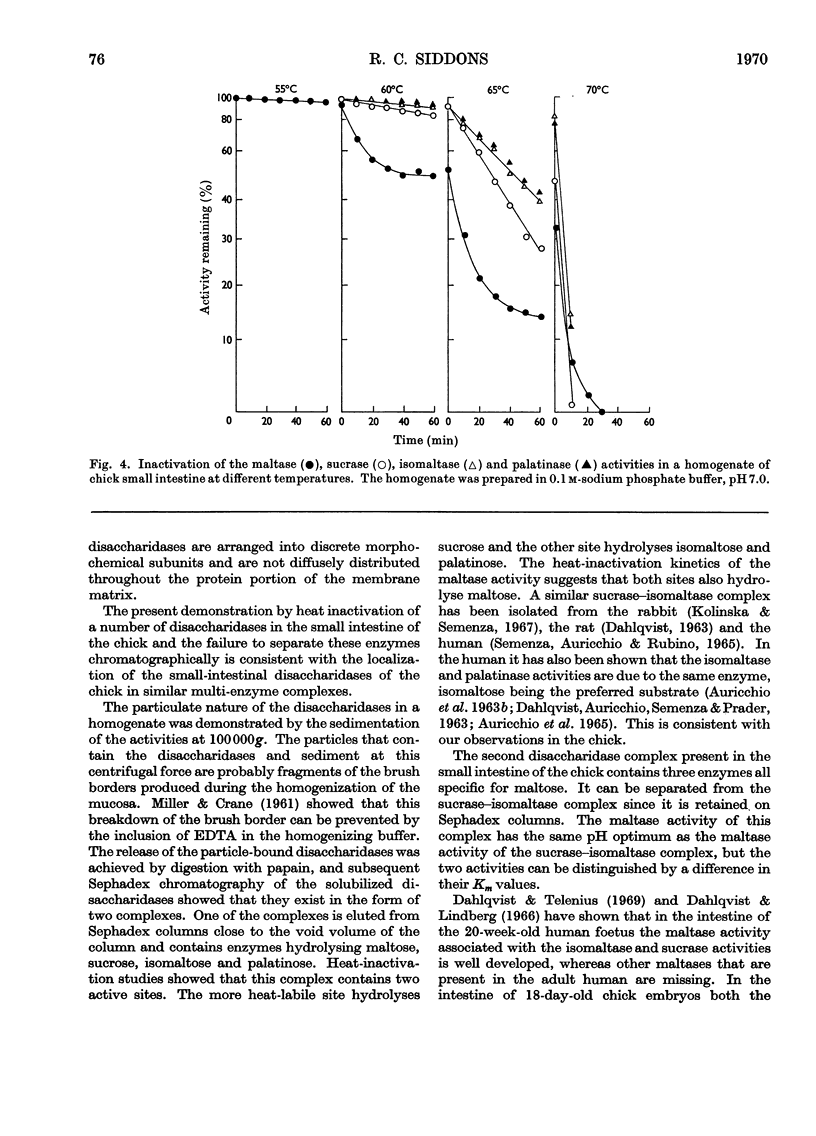
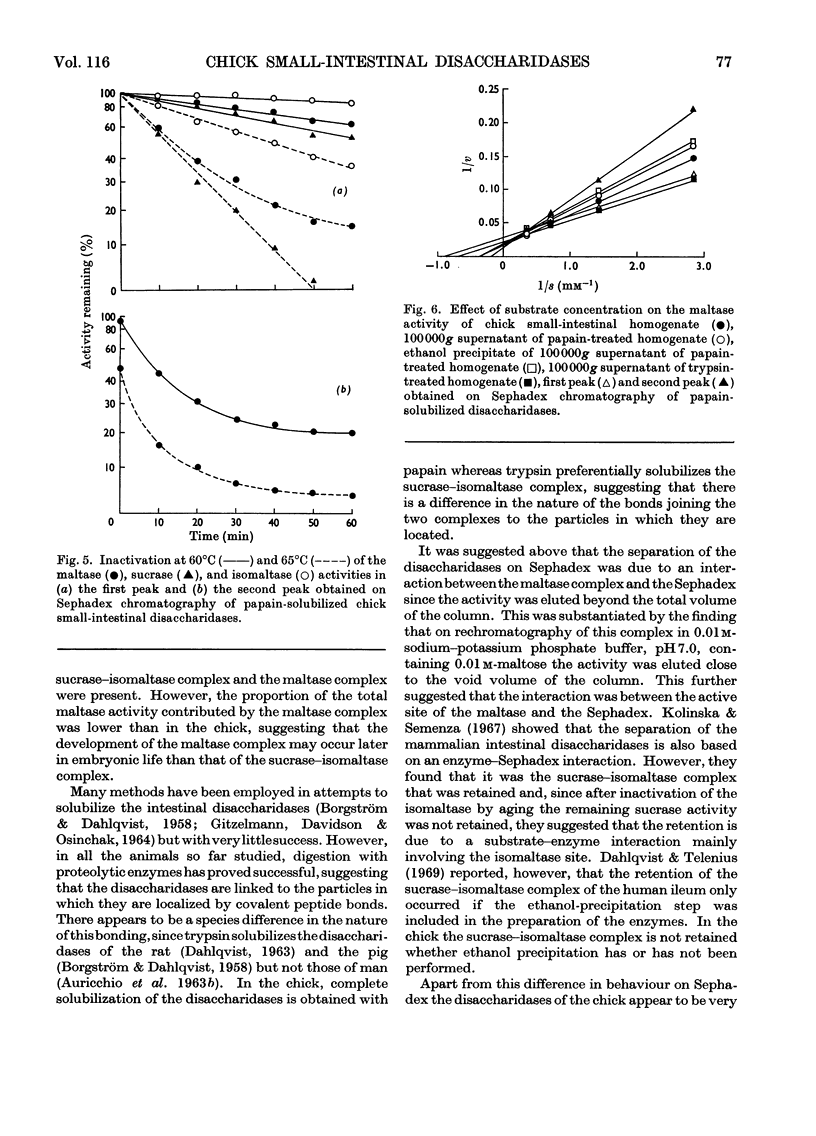
1. The maltase, sucrase, isomaltase and palatinase activities of the chick small intestine are localized in particles that sediment when centrifuged at 100000g for 90min. 2. Solubilization of the particle-bound disaccharidases without loss of activity was achieved by digestion with papain. Trypsin was less effective and caused a preferential solubilization of the sucrase, isomaltase and palatinase activities. 3. On Sephadex G-200 columns, the solubilized preparations yielded two disaccharidase peaks. The first peak was eluted close to the void volume of the column and contained all the sucrase, isomaltase and palatinase activities and some of the maltase activity. The remainder of the maltase activity was eluted beyond the total volume of the column. 4. Precipitation with ethanol did not affect the behaviour of the disaccharidases of gel filtration. 5. The maltase activity of the second peak on rechromatography in a buffer containing 0.01m-maltose was eluted close to the void volume. 6. Similar pH optima but different Km values were obtained for the maltase activities of the two peaks. 7. Heat-inactivation studies showed that the first peak contained two disaccharidase enzymes; one hydrolysed sucrose and maltose and the other hydrolysed isomaltose, palatinose and maltose. The second peak contained three disaccharidase enzymes all specific for the hydrolysis of maltose. 8. It is proposed that the intestinal disaccharidases of the chick exist in the form of two complexes: a sucrase–isomaltase complex and a maltase complex.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AURICCHIO S., DAHLQVIST A., SEMENZA G. SOLUBILIZATION OF THE HUMAN INTESTINAL DISACCHARIDASES. Biochim Biophys Acta. 1963 Aug 6;73:582–587. doi: 10.1016/0006-3002(63)90329-9. [DOI] [PubMed] [Google Scholar]
- AURICCHIO S., SEMENZA G., RUBINO A. MULTIPLICITY OF HUMAN INTESTINAL DISACCHARIDASES. II. CHARACTERIZATION OF THE INDIVIDUAL MALTASES. Biochim Biophys Acta. 1965 Mar 22;96:498–507. doi: 10.1016/0005-2787(65)90566-6. [DOI] [PubMed] [Google Scholar]
- DAHLQVIST A., AURICCHIO S., SEMENZA G., PRADER A. Human intestinal disaccharidases and hereditary disaccharide intolerance. The hydrolysis of sucrose, isomaltose, palatinose (isomaltulose), and a 1,6-alpha-oligosaccharide (isomalto-oligosaccharide) preparation. J Clin Invest. 1963 Apr;42:556–562. doi: 10.1172/JCI104744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAHLQVIST A. METHOD FOR ASSAY OF INTESTINAL DISACCHARIDASES. Anal Biochem. 1964 Jan;7:18–25. doi: 10.1016/0003-2697(64)90115-0. [DOI] [PubMed] [Google Scholar]
- DAHLQVIST A. Rat-intestinal dextranase. Localization and relation to the other carbohydrases of the digestive tract. Biochem J. 1963 Jan;86:72–76. doi: 10.1042/bj0860072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlqvist A., Lindberg T. Development of the intestinal disaccharidase and alkaline phosphatase activities in the human foetus. Clin Sci. 1966 Jun;30(3):517–528. [PubMed] [Google Scholar]
- Dahlqvist A., Telenius U. Column chromatography of human small-intestinal maltase, isomaltase and invertase activities. Biochem J. 1969 Jan;111(2):139–146. doi: 10.1042/bj1110139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichholz A. Studies on the organization of the brush border in intestinal epithelial cells. V. Subfractionation of enzymatic activities of the microvillus membrane. Biochim Biophys Acta. 1968 Aug;163(1):101–107. doi: 10.1016/0005-2736(68)90037-0. [DOI] [PubMed] [Google Scholar]
- GITZELMANN R., DAVIDSON E. A., OSINCHAK J. DISACCHARIDASE OF RABBIT SMALL INTESTINE: INTRACELLULAR DISTRIBUTION, SOLUBILIZATION, PURIFICATION AND SPECIFICITY. Biochim Biophys Acta. 1964 Apr 6;85:69–81. doi: 10.1016/0926-6569(64)90168-3. [DOI] [PubMed] [Google Scholar]
- Johnson C. F. Disaccharidase: localization in hamster intestine brush borders. Science. 1967 Mar 31;155(3770):1670–1672. doi: 10.1126/science.155.3770.1670. [DOI] [PubMed] [Google Scholar]
- Jos J., Frezal J., Rey J., Lamy M. Histochemical localization of intestinal disaccharidases: application to peroral biopsy specimens. Nature. 1967 Feb 4;213(5075):516–518. doi: 10.1038/213516a0. [DOI] [PubMed] [Google Scholar]
- Kolínská J., Semenza G. Studies on intestinal sucrase and on intestinal sugar transport. V. Isolation and properties of sucrase-isomaltase from rabbit small intestine. Biochim Biophys Acta. 1967 Sep 12;146(1):181–195. doi: 10.1016/0005-2744(67)90085-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MILLER D., CRANE R. K. The digestive function of the epithelium of the small intestine. I. An intracellular locus of disaccharide and sugar phosphate ester hydrolysis. Biochim Biophys Acta. 1961 Sep 16;52:281–293. doi: 10.1016/0006-3002(61)90677-1. [DOI] [PubMed] [Google Scholar]
- SEMENZA G., AURICCHIO S., RUBINO A. MULTIPLICITY OF HUMAN INTESTINAL DISACCHARIDASES. I. CHROMATOGRAPHIC SEPARATION OF MALTASES AND OF TWO LACTASES. Biochim Biophys Acta. 1965 Mar 22;96:487–497. doi: 10.1016/0005-2787(65)90565-4. [DOI] [PubMed] [Google Scholar]
- SEMENZA G., TOSI R., VALLOTTON-DELACHAUX M. C., MUELHAUPT E. SODIUM ACTIVATION OF HUMAN INTESTINAL SUCRASE AND ITS POSSIBLE SIGNIFICANCE IN THE ENZYME ORGANIZATION OF BRUSH BORDERS. Biochim Biophys Acta. 1964 Jul 8;89:109–116. doi: 10.1016/0926-6569(64)90104-x. [DOI] [PubMed] [Google Scholar]
- Siddons R. C. Intestinal disaccharidase activities in the chick. Biochem J. 1969 Mar;112(1):51–59. doi: 10.1042/bj1120051. [DOI] [PMC free article] [PubMed] [Google Scholar]


