Abstract
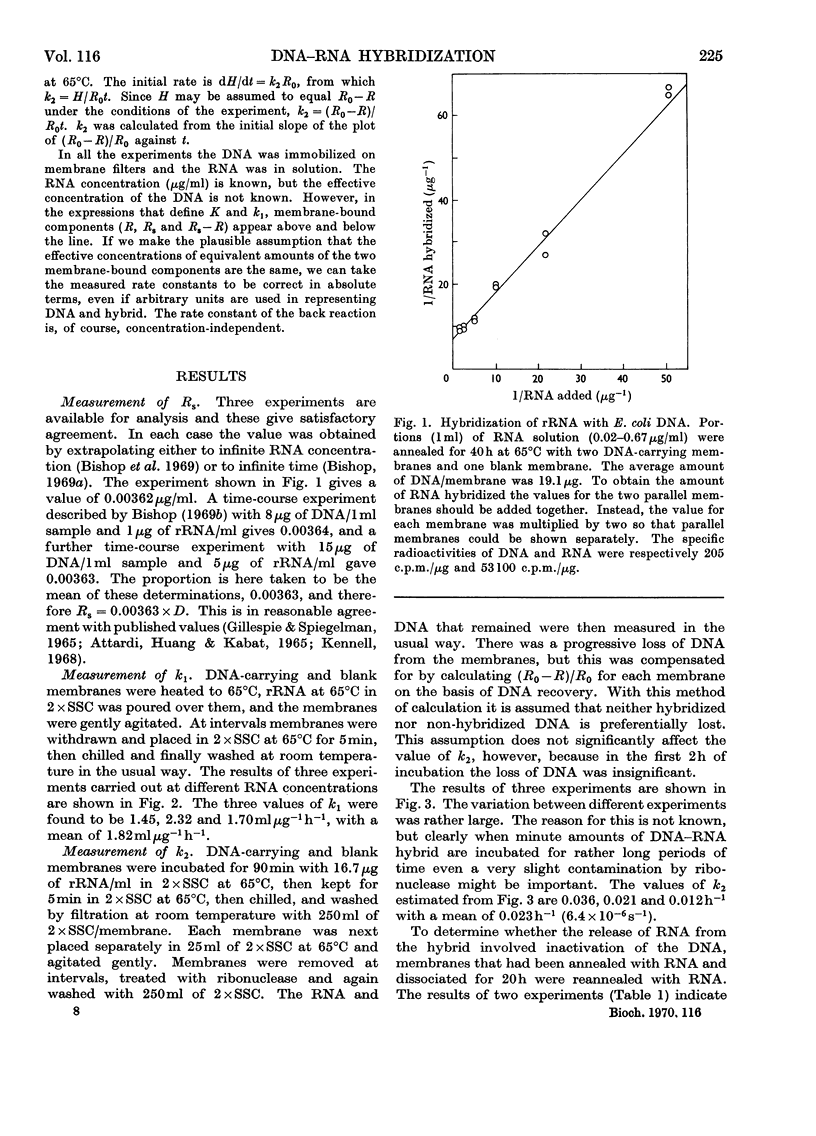
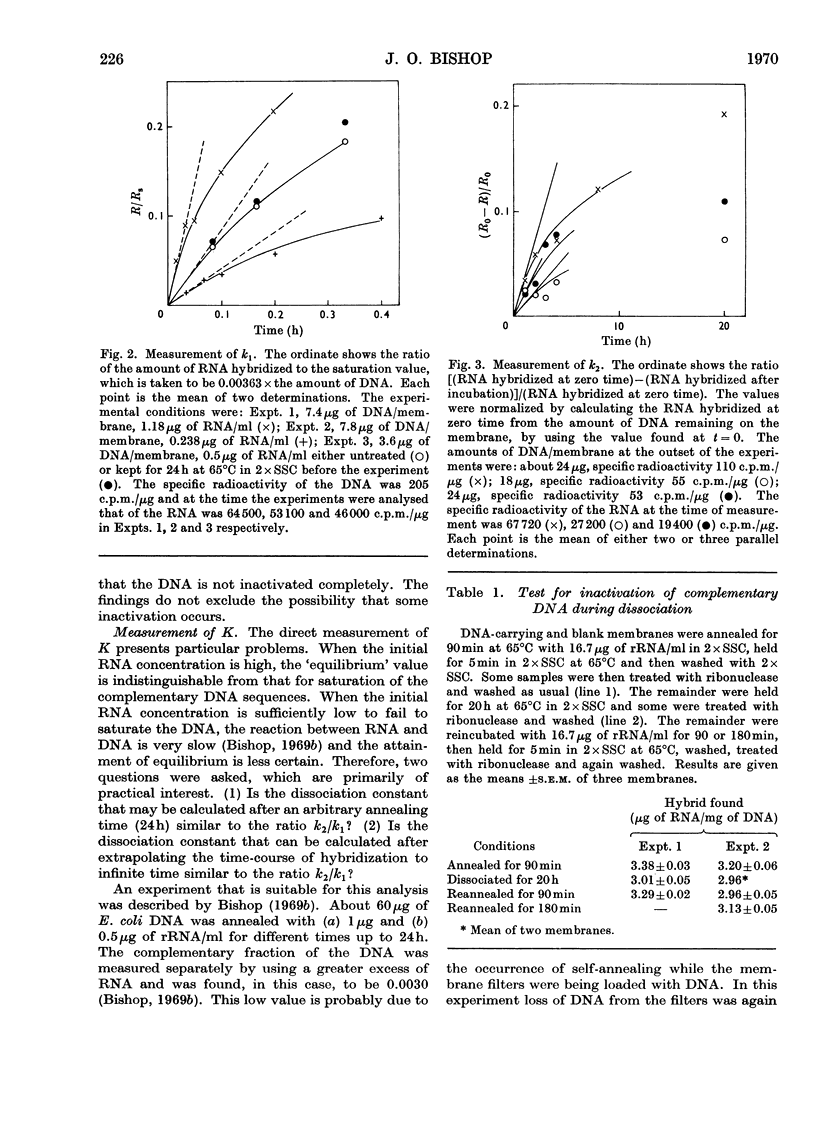
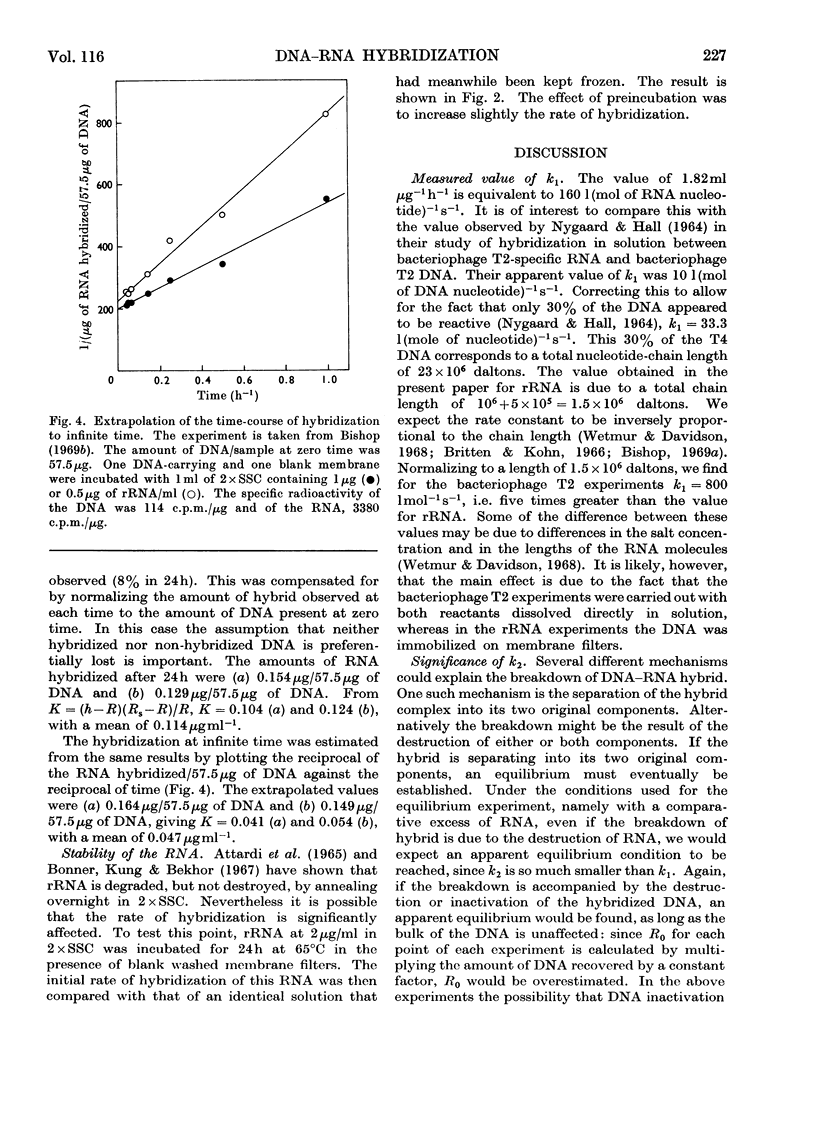
1. When a constant amount of denatured DNA is annealed for a constant time with a series of different RNA concentrations, it is often observed that the reciprocal of the amount of RNA hybridized is linearly proportional to the reciprocal of the RNA concentration. This may be explained by assuming that an equilibrium is set up between free RNA and DNA on the one hand and DNA–RNA hybrid on the other. The hybridization of Escherichia coli DNA and ribosomal RNA was used to test this proposition. Rate constants were estimated from the initial rates of the forward and back reactions and compared with direct estimates of the dissociation constant. 2. The rate constants of the forward and back reactions were estimated to be 1.82mlμg−1h−1 (160lmol−1s−1) and 0.023h−1 (6.4×10−6s−1) respectively, giving a ratio k2/k1=0.013μgml−1. After 24h annealing the dissociation constant was estimated to be 0.114μgml−1, and by extrapolation to infinite time, 0.047μgml−1. 3. It is concluded that (a) equilibrium greatly favours the hybrid complex, (b) equilibrium is not established in 24h, (c) the equilibria that were directly estimated are incompatible either with the measured rates of the forward and back reactions or with the simple formulation of the reaction that was adopted, and finally (d) for these reasons the equilibrium interpretation of the linear reciprocal relationship is unsatisfactory.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attardi G., Huang P. C., Kabat S. Recognition of ribosomal RNA sites in DNA. I. Analysis of the E. coli system. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1490–1498. doi: 10.1073/pnas.53.6.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. O. Interpretation of DNA-RNA hybridization data. Nature. 1969 Nov 8;224(5219):600–603. doi: 10.1038/224600a0. [DOI] [PubMed] [Google Scholar]
- Bishop J. O., Robertson F. W., Burns J. A., Melli M. Methods for the analysis of deoxyribonucleic acid-ribonucleic acid hybridization data. Biochem J. 1969 Nov;115(3):361–370. doi: 10.1042/bj1150361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. O. The effect of genetic complexity on the time-course of ribonucleic acid-deoxyribonucleic acid hybridization. Biochem J. 1969 Aug;113(5):805–811. doi: 10.1042/bj1130805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner J., Kung G., Bekhor I. A method for the hybridization of nucleic acid molecules at low temperature. Biochemistry. 1967 Dec;6(12):3650–3653. doi: 10.1021/bi00864a005. [DOI] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- HALL B. D., SPIEGELMAN S. Sequence complementarity of T2-DNA and T2-specific RNA. Proc Natl Acad Sci U S A. 1961 Feb 15;47:137–163. doi: 10.1073/pnas.47.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NYGAARD A. P., HALL B. D. FORMATION AND PROPERTIES OF RNA-DNA COMPLEXES. J Mol Biol. 1964 Jul;9:125–142. doi: 10.1016/s0022-2836(64)80095-4. [DOI] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]
- YANKOFSKY S. A., SPIEGELMAN S. The identification of the ribosomal RNA cistron by sequence complementarity. II. Saturation of and competitive interaction at the RNA cistron. Proc Natl Acad Sci U S A. 1962 Aug;48:1466–1472. doi: 10.1073/pnas.48.8.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]


