Abstract
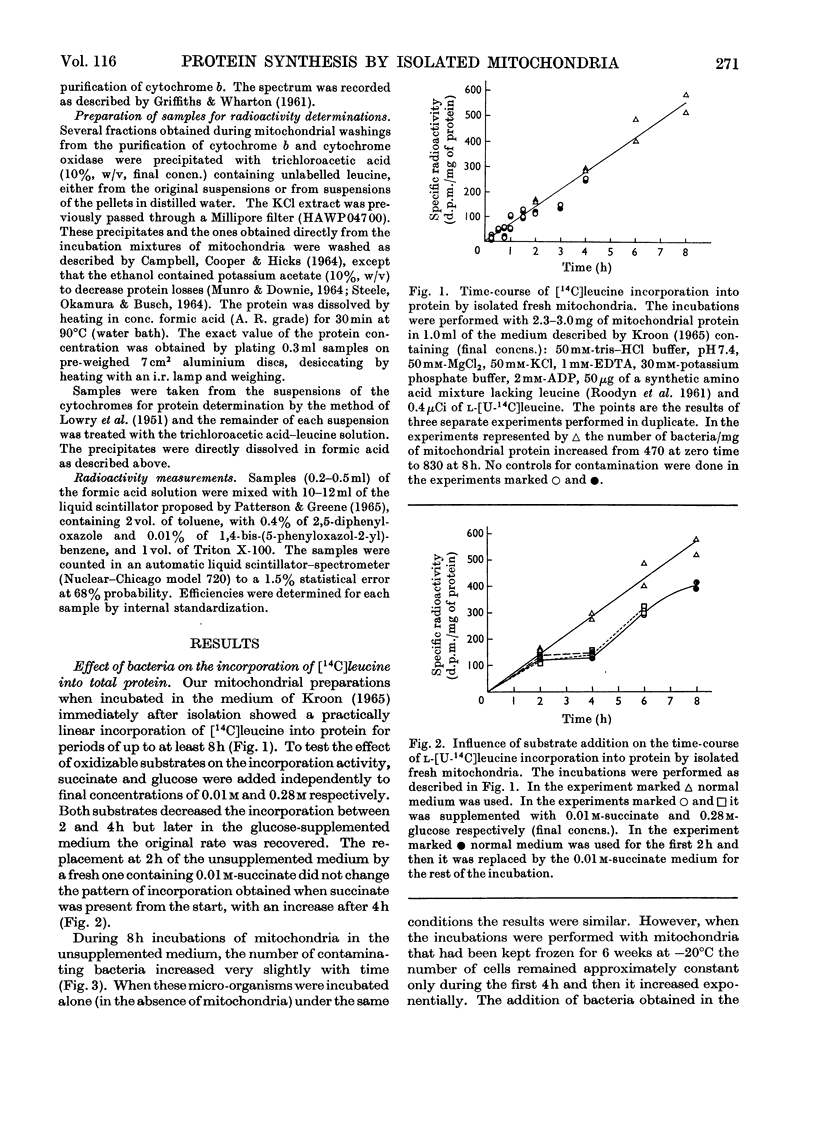
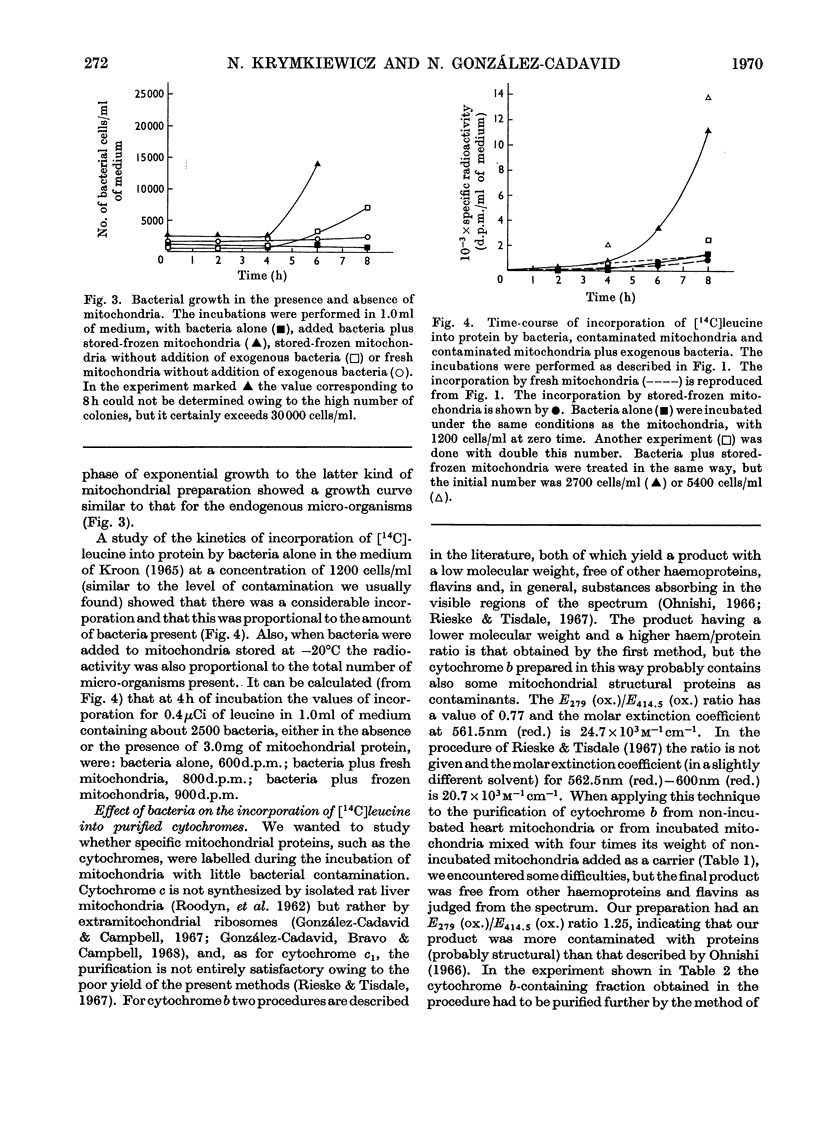
The problem of whether isolated mitochondria are able to synthesize specific proteins was investigated, particular consideration being paid to the possible contribution of micro-organisms to this activity. With ox heart mitochondria it was shown that: (1) The medium used for the incubations inhibits the exponential phase of bacterial growth for at least 8h either in the absence or the presence of fresh mitochondria, but the inhibition disappears after 4h when mitochondria damaged by freezing and thawing are used. (2) The incorporation of [14C]leucine into total proteins is linear up to at least 8h, although part of the radioactivity at the later periods might be due to some incorporation by resting-phase bacteria. (3) A contamination by as little as 800 cells/mg of mitochondrial protein is enough to contribute substantially to the total radioactivity incorporated by the mitochondrial preparations. (4) Purified cytochrome b and cytochrome oxidase are labelled even under conditions of minimal contamination by micro-organisms (less than 60 cells/mg of mitochondrial protein) and the contribution of bacterial proteins to the radioactivity found in cytochromes is negligible, as shown by double-labelling experiments. (5) At 4h the specific radioactivities of cytochrome b and cytochrome oxidase are seven- and 16-fold lower respectively than that of a structural protein-rich fraction, suggesting that the labelling of cytochromes is due to a residual contamination by these proteins.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beattie D. S., Basford R. E., Koritz S. B. Bacterial contamination and amino acid incorporation by isolated mitochondria. J Biol Chem. 1967 Jul 25;242(14):3366–3368. [PubMed] [Google Scholar]
- Campbell P. N., Cooper C., Hicks M. Studies on the role of the morphological constituents of the microsome fraction from rat liver in protein synthesis. Biochem J. 1964 Aug;92(2):225–234. doi: 10.1042/bj0920225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criddle R. S., Schatz G. Promitochondria of anaerobically grown yeast. I. Isolation and biochemical properties. Biochemistry. 1969 Jan;8(1):322–334. doi: 10.1021/bi00829a045. [DOI] [PubMed] [Google Scholar]
- GRIFFITHS D. E., WHARTON D. C. Studies of the electron transport system. XXXV. Purification and properties of cytochrome oxidase. J Biol Chem. 1961 Jun;236:1850–1856. [PubMed] [Google Scholar]
- González-Cadavid N. F., Bravo M., Campbell P. N. The significance of cytochrome c redistribution during the subcellular fractionation of rat liver. Biochem J. 1968 Apr;107(4):523–529. doi: 10.1042/bj1070523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Cadavid N. F., Campbell P. N. The biosynthesis of cytochrome c. Sequence of incorporation in vivo of [14C]lysine into cytochrome c and total proteins of rat-liver subcellular fractions. Biochem J. 1967 Nov;105(2):443–450. doi: 10.1042/bj1050443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. E., Haard N. F., Lenaz G., Silman H. I. On the noncatalytic proteins of membrane systems. Proc Natl Acad Sci U S A. 1968 May;60(1):277–284. doi: 10.1073/pnas.60.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar D., Freeman K. B. Importance of the osmolarity of the incubation medium on amino acid incorporation into protein by isolated rat liver mitochondria. Biochem J. 1969 Mar;111(5):653–661. doi: 10.1042/bj1110653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar D., Freeman K., Work T. S. Biogenesis ommitochondria. Nature. 1966 Jul 2;211(5044):9–12. doi: 10.1038/211009a0. [DOI] [PubMed] [Google Scholar]
- Henson C. P., Weber C. N., Mahler H. R. Formation of yeast mitochondria. I. Kinetics of amino acid incorporation during derepression. Biochemistry. 1968 Dec;7(12):4431–4444. doi: 10.1021/bi00852a040. [DOI] [PubMed] [Google Scholar]
- Kroon A. M. Protein synthesis in mitochondria. 3. On the effects of inhibitors on the incorporation of amino acids into protein by intact mitochondria and digitonin fractions. Biochim Biophys Acta. 1965 Oct 11;108(2):275–284. doi: 10.1016/0005-2787(65)90012-2. [DOI] [PubMed] [Google Scholar]
- Kroon A. M., Saccone C., Botman M. J. RNA and protein synthesis by sterile rat-liver mitochondria. Biochim Biophys Acta. 1967 Jul 18;142(2):552–554. doi: 10.1016/0005-2787(67)90639-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McLEAN J. R., COHN G. L., BRANDT I. K., SIMPSON M. V. Incorporation of labeled amino acids into the protein of muscle and liver mitochondria. J Biol Chem. 1958 Sep;233(3):657–663. [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- ROODYN D. B., FREEMAN K. B., TATA J. R. THE STIMULATION BY TREATMENT IN VIVO WITH TRI-IODOTHYRONINE OF AMINO ACID INCORPORATION INTO PROTEIN BY ISOLATED RAT-LIVER MITOCHONDRIA. Biochem J. 1965 Mar;94:628–641. doi: 10.1042/bj0940628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROODYN D. B. Protein synthesis in mitochondria. 3. The controlled disruption and subfractionation of mitochondria labelled in vitro with radioactive valine. Biochem J. 1962 Oct;85:177–189. doi: 10.1042/bj0850177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROODYN D. B., REIS P. J., WORK T. S. Protein synthesis in mitochondria. Requirements for the incorporation of radioactive amino acids into mitochondrial protein. Biochem J. 1961 Jul;80:9–21. doi: 10.1042/bj0800009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROODYN D. B., SUTTIE J. W., WORK T. S. Protein synthesis in mitochondria. 2. Rate of incorporation in vitro of radioactive amino acids into soluble proteins in the mitochondrial fraction, including catalase, malic dehydrogenase and cytochrome c. Biochem J. 1962 Apr;83:29–40. doi: 10.1042/bj0830029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roodyn D. B. Further study of factors affecting amino acid incorporation into protein by isolated mitochondria. Biochem J. 1965 Dec;97(3):782–793. doi: 10.1042/bj0970782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEELE W. J., OKAMURA N., BUSCH H. PREVENTION OF LOSS OF RNA, DNA AND PROTEIN INTO LIPID SOLVENTS. Biochim Biophys Acta. 1964 Jul 22;87:490–492. doi: 10.1016/0926-6550(64)90120-3. [DOI] [PubMed] [Google Scholar]
- Sandell S., Löw H., von der Decken A. A critical study of amino acid incorporation into protein by isolated liver mitochondria from adult rats. Biochem J. 1967 Aug;104(2):575–585. doi: 10.1042/bj1040575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeldon L. W., Lehninger A. L. Energy-linked synthesis and decay of membrane proteins in isolated rat liver mitochondria. Biochemistry. 1966 Nov;5(11):3533–3545. doi: 10.1021/bi00875a021. [DOI] [PubMed] [Google Scholar]
- Wheeldon L. The problem of bacterial contamination in studies of protein synthesis by isolated mitochondria. Biochem Biophys Res Commun. 1966 Aug 12;24(3):407–411. doi: 10.1016/0006-291x(66)90174-4. [DOI] [PubMed] [Google Scholar]
- Work T. S., Coote J. L., Ashwell M. Biogenesis of mitochondria. Fed Proc. 1968 Sep-Oct;27(5):1174–1179. [PubMed] [Google Scholar]
- van Dam K., Tsou C. S. Accumulation of substrates by mitochondria. Biochim Biophys Acta. 1968 Oct 1;162(3):301–309. doi: 10.1016/0005-2728(68)90116-3. [DOI] [PubMed] [Google Scholar]


