Abstract
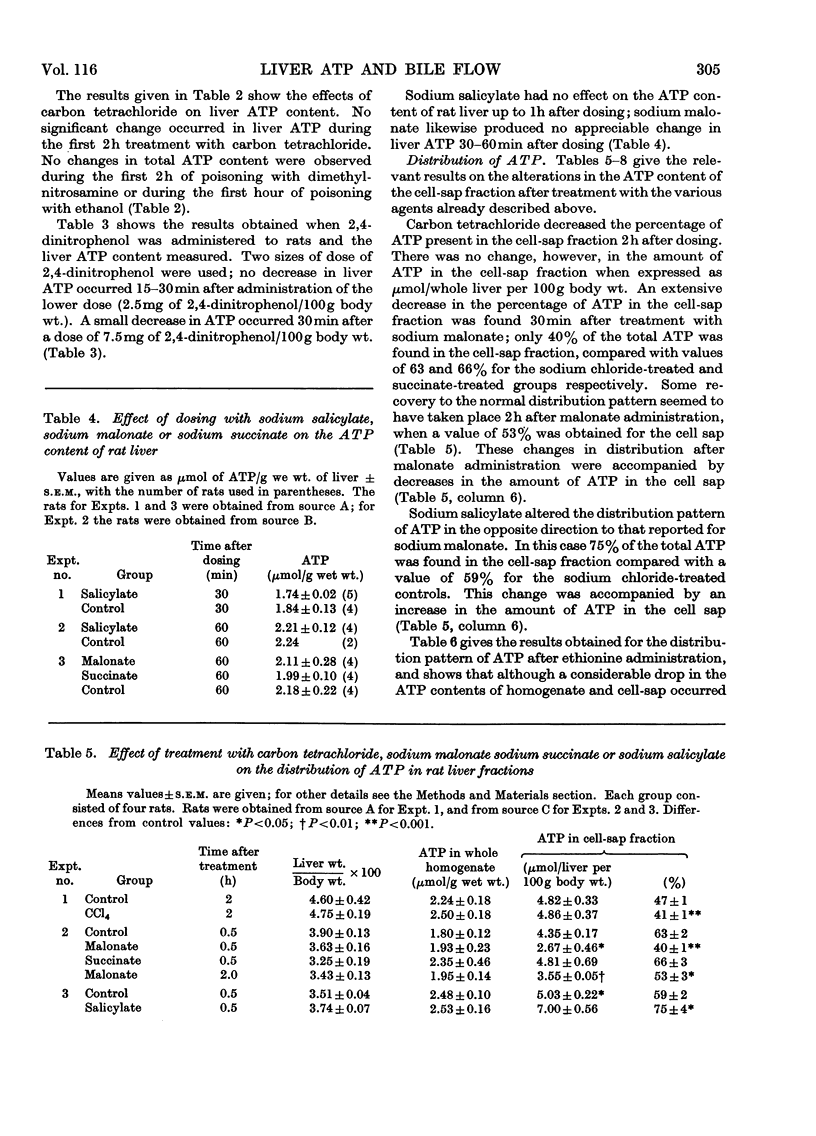
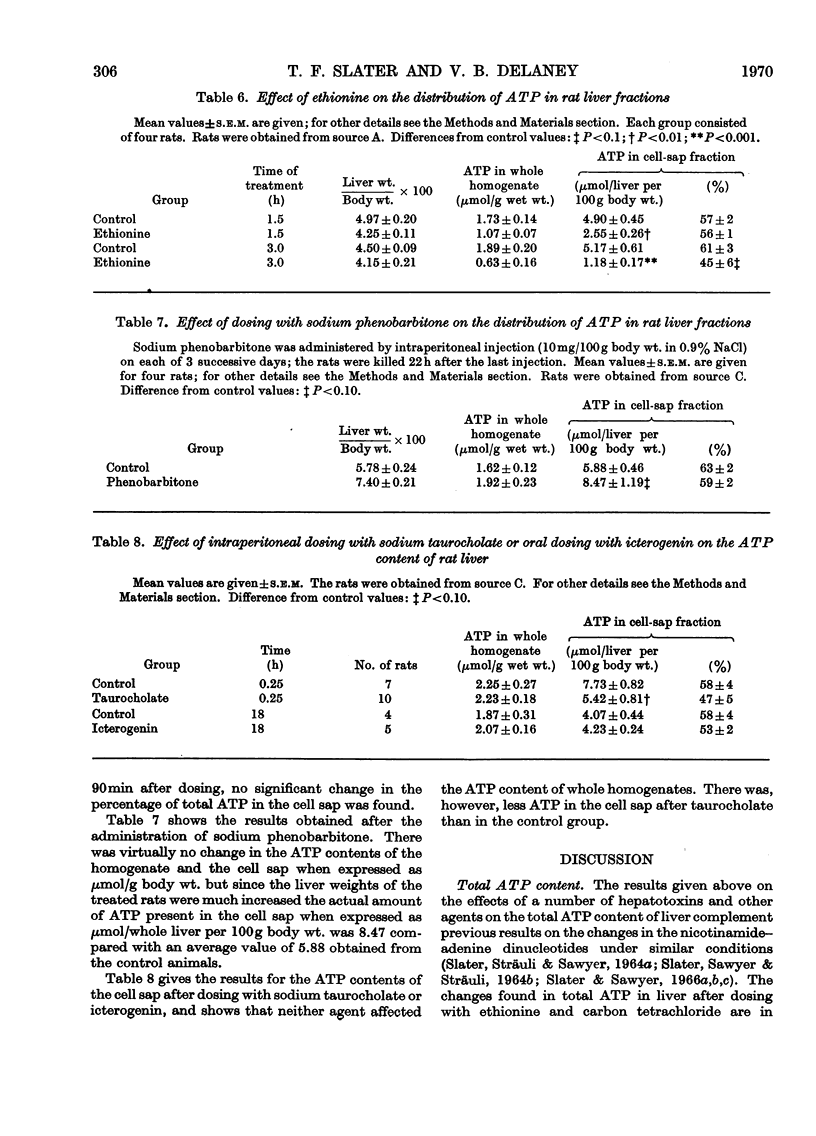
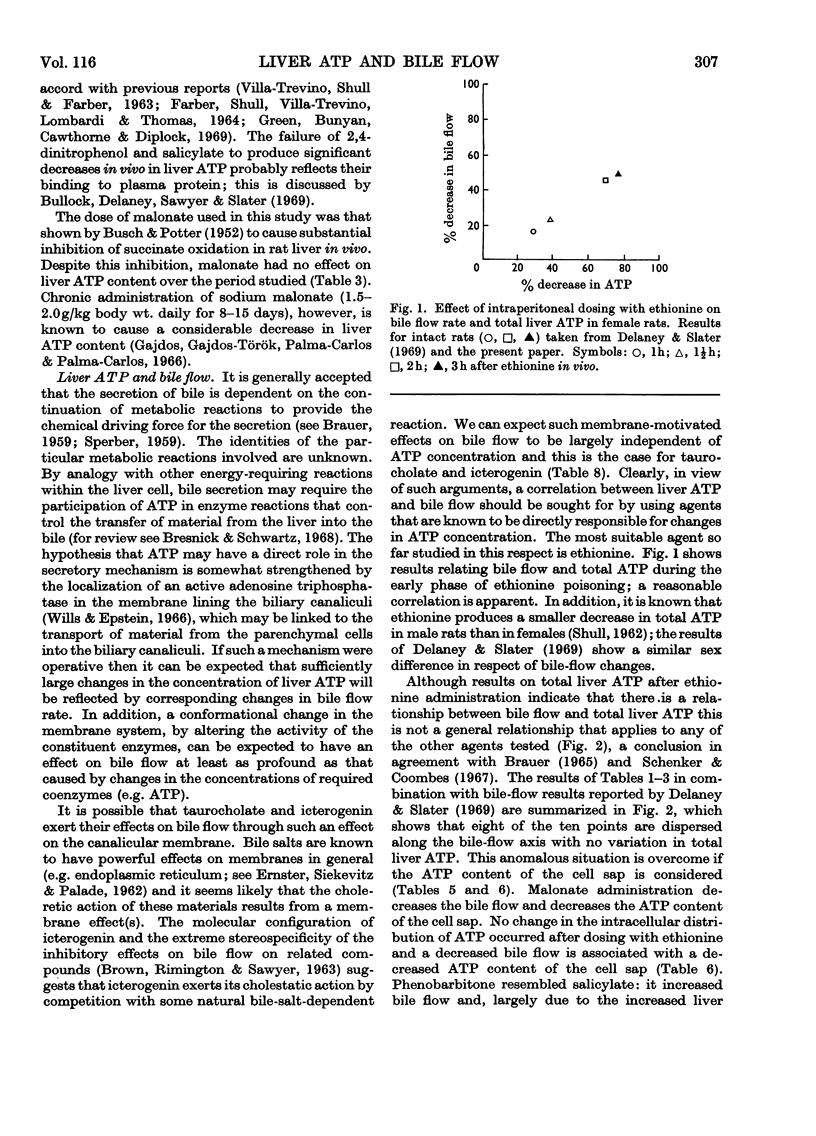
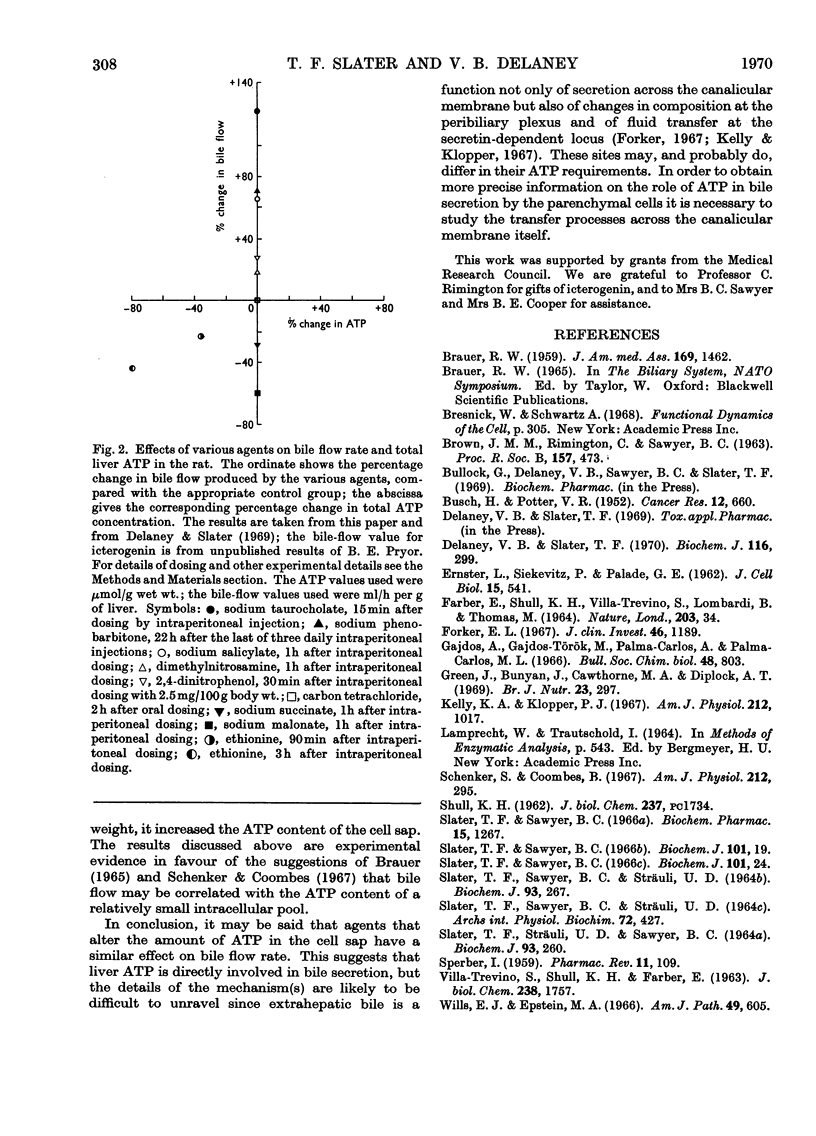
The effects of a number of hepatotoxic and other agents on the ATP content of rat liver are described. Changes in the distribution of ATP between the cell sap and the large-particle fraction were determined at intervals after rats had been dosed with various substances. Ethionine produced a rapid decrease in total liver ATP but no alteration in its intracellular distribution. Carbon tetrachloride, sodium salicylate, dimethylnitrosamine, 2,4-dinitrophenol, icterogenin, sodium succinate, sodium malonate and sodium taurocholate did not significantly alter the total ATP content of liver in the periods studied but changes in intracellular distribution were found. Carbon tetrachloride, malonate and taurocholate decreased, and salicylate treatment increased, the proportion of ATP in the cell sap. Treatment with sodium phenobarbitone increased the total liver ATP and the total amount of ATP in the cell sap. The changes in ATP concentration and in the intracellular distribution of ATP are correlated with changes previously reported in bile flow (Delaney & Slater, 1969). No general correlation was found between changes in total ATP and changes in bile flow rate, but there was a relationship between changes in bile flow and in ATP content in the case of ethionine. With the exception of taurocholate and icterogenin, which possibly act on a membrane site, an approximate correlation was found between changes in bile flow and changes in the amount of ATP in the cell sap. The findings are discussed in terms of possible mechanisms for biliary secretion.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAUER R. W. Mechanisms of bile secretion. J Am Med Assoc. 1959 Mar 28;169(13):1462–1466. doi: 10.1001/jama.1959.03000300058011. [DOI] [PubMed] [Google Scholar]
- BROWN J. M., RIMINGTON C., SAWYER B. C. Studies on biliary excretion in the rabbit. II. The relationship between the chemical structure of certain natural or synthetic pentacyclic triterpenes and their icterogenic activity. 1. The substituents on carbon atoms 3, 17, 22 and 24. Proc R Soc Lond B Biol Sci. 1963 May 28;157:473–491. doi: 10.1098/rspb.1963.0023. [DOI] [PubMed] [Google Scholar]
- BUSCH H., POTTER V. R. Studies on tissue metabolism by means of in vivo metabolic blocking technics. I. A survey of changes induced by malonate in tissues of tumor-bearing rats. Cancer Res. 1952 Sep;12(9):660–665. [PubMed] [Google Scholar]
- Delaney V. B., Slater T. F. A filtration method for the separation of cell sap from the large-particle fraction of rat liver suspensions, enabling the rapid measurement of nucleotide distributions. Biochem J. 1970 Jan;116(2):299–302. doi: 10.1042/bj1160299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARBER E., SHULL K. H., VILLA-TREVINO S., LOMBARDI B., THOMAS M. BIOCHEMICAL PATHOLOGY OF ACUTE HEPATIC ADENOSINETRIPHOSPHATE DEFICIENCY. Nature. 1964 Jul 4;203:34–40. doi: 10.1038/203034a0. [DOI] [PubMed] [Google Scholar]
- Forker E. L. Two sites of bile formation as determined by mannitol and erythritol clearance in the guinea pig. J Clin Invest. 1967 Jul;46(7):1189–1195. doi: 10.1172/JCI105612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajdos A., Gajdos-Törok M., Palma Carlos A., Palma Carlos M. L. Abaissement du taux hépatique de l'ATP au cours de la porphyrie provoquée chez le rat par administration de malonate de sodium. Bull Soc Chim Biol (Paris) 1966;48(6):803–814. [PubMed] [Google Scholar]
- Green J., Bunyan J., Cawthorne M. A., Diplock A. T. Vitamin E and hepatotoxic agents. 1. Carbon tetrachloride and lipid peroxidation in the rat. Br J Nutr. 1969 Jun;23(2):297–307. doi: 10.1079/bjn19690037. [DOI] [PubMed] [Google Scholar]
- SPERBER I. Secretion of organic anions in the formation of urine and bile. Pharmacol Rev. 1959 Mar;11(1):109–134. [PubMed] [Google Scholar]
- Slater T. F., Sawyer B. C. Liver nucleotides in acute experimental liver injury induced by dimethylnitrosamine and by thioacetamide. Biochem J. 1966 Oct;101(1):19–23. doi: 10.1042/bj1010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater T. F., Sawyer B. C. Nicotinamide-adenine dinucleotides in acute liver injury induced by ethionine, and a comparison with the effects of salicylate. Biochem J. 1966 Oct;101(1):24–28. doi: 10.1042/bj1010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater T. F., Sawyer B. C., Sträuli U. D. Changes in liver nucleotide concentrations in experimental liver injury. 2. Acute ethanol poisoning. Biochem J. 1964 Nov;93(2):267–270. doi: 10.1042/bj0930267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater T. F., Sawyer B., Sträuli U. An assay procedure for nicotinamide-adenine dinucleotides in rat liver and other tissues. Arch Int Physiol Biochim. 1964 Jun;72(3):427–447. doi: 10.3109/13813456409065351. [DOI] [PubMed] [Google Scholar]
- Slater T. F., Sträuli U. D., Sawyer B. C. Changes in liver nucleotide concentrations in experimental liver injury. 1. Carbon tetrachloride poisoning. Biochem J. 1964 Nov;93(2):260–266. doi: 10.1042/bj0930260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VILLA-TREVINO S., SHULL K. H., FARBER E. The role of adenosine triphosphate deficiency in ethionine-induced inhibition of protein synthesis. J Biol Chem. 1963 May;238:1757–1763. [PubMed] [Google Scholar]
- Wills E. J., Epstein M. A. Subcellular changes in surface adenosine triphosphatase activity of human liver in extrahepatic obstructive jaundice. Am J Pathol. 1966 Oct;49(4):605–635. [PMC free article] [PubMed] [Google Scholar]


