Abstract
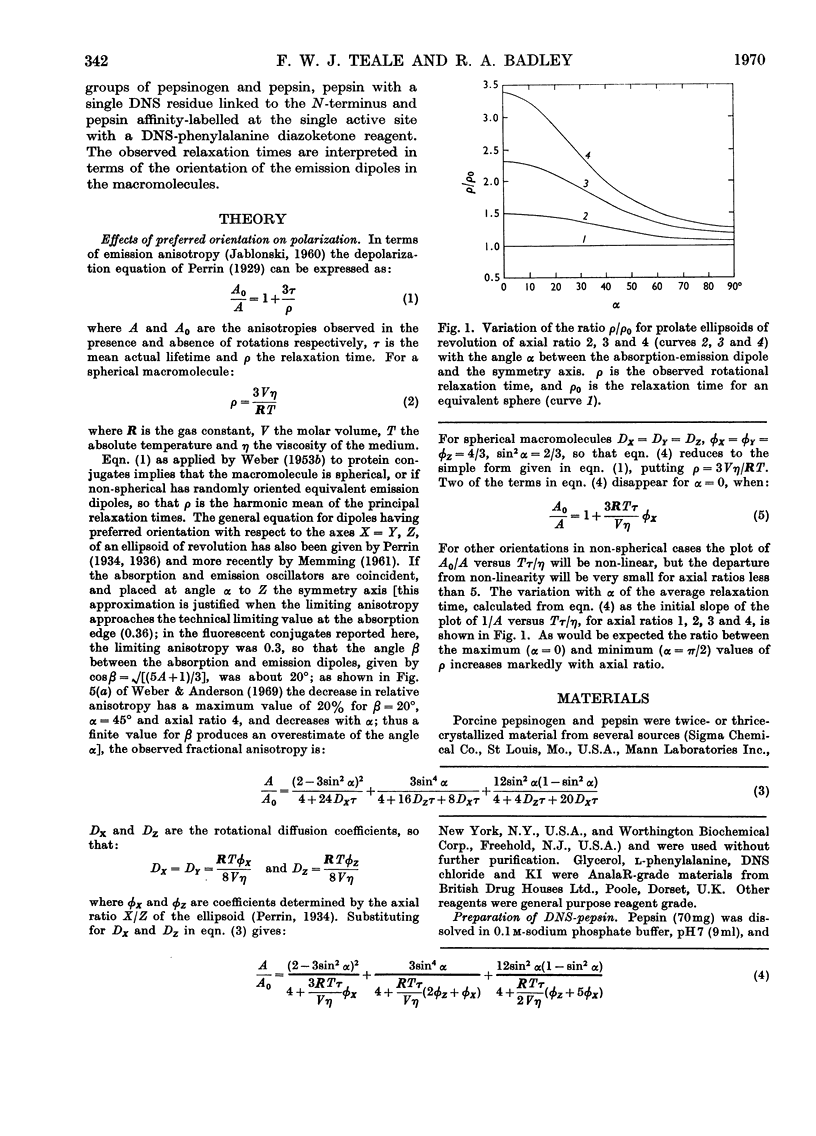
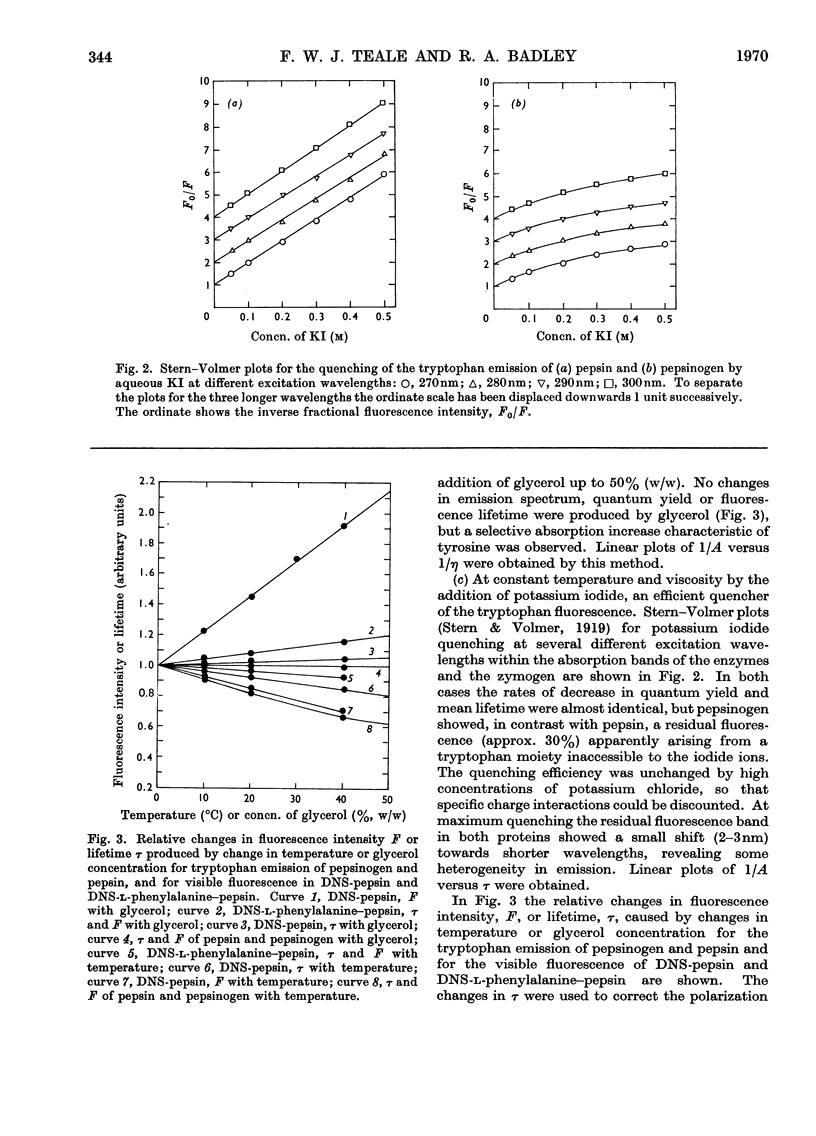
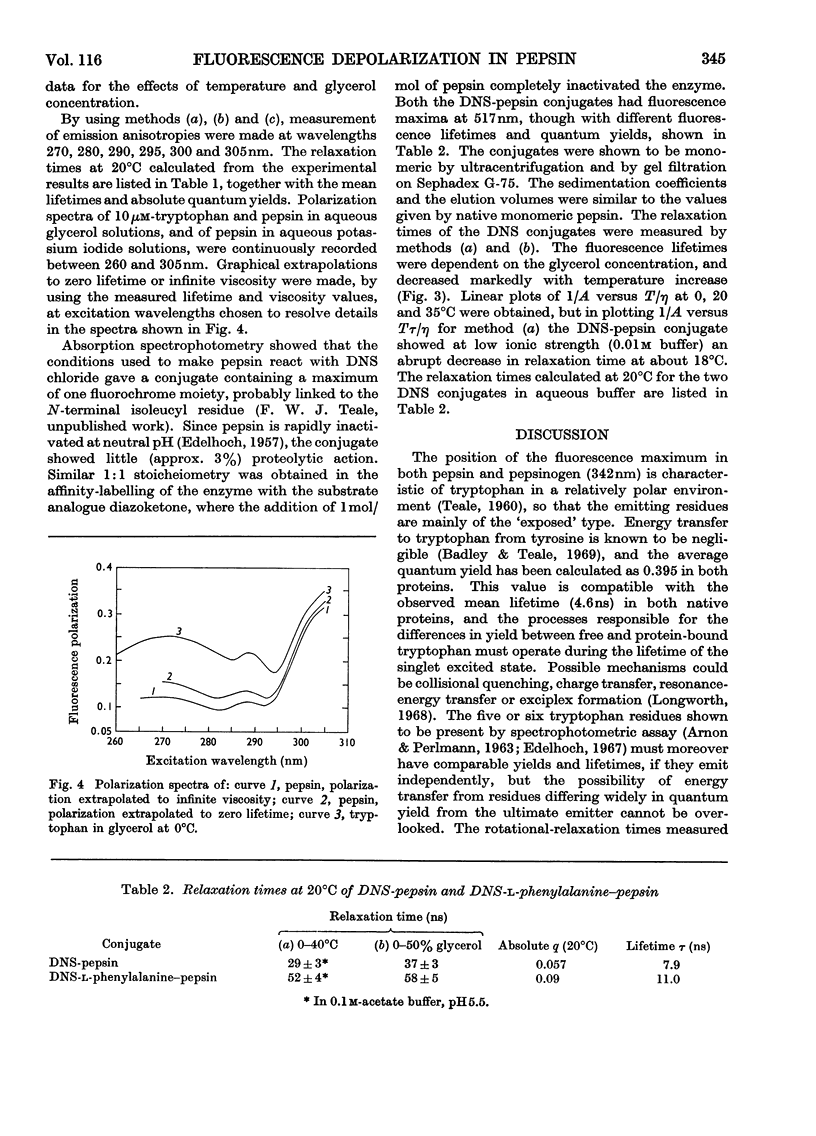
1. The effects on the intrinsic tryptophan emission anisotropy of pepsin and pepsinogen solutions produced by (a) changes in temperature, (b) increases in viscosity with added glycerol at constant temperature and (c) decreases in lifetime through collisional quenching by potassium iodide were measured at several excitation wavelengths. The rotational-relaxation times calculated from results provided by method (b) approximate to the theoretical values for the two proteins, on taking hydration and shape factors into account, on the basis of random orientation of the tryptophan groups within the macromolecules. Differences between the results provided by methods (b) and (c) are attributable to inter-tryptophan resonance-energy-transfer depolarization, and the anomalous values recorded in method (a) can be attributed to the temperature-dependence of the limiting anisotropies. 2. Two different monomeric conjugates of pepsin, each containing one extrinsic fluorescent group per macromolecule, gave widely different relaxation times. This difference may arise from a specific orientation of the emission dipole in the enzyme. In active-site-labelled pepsin (1-dimethylaminonaphthalene-5-sulphonylphenylalanine–pepsin) this orientation would be approximately parallel to the symmetry axis of the equivalent ellipsoid, whereas in the other conjugate (1-dimethylaminonaphthalene-5-sulphonyl-pepsin) the orientation may be roughly normal to this direction, or some independent rotation of parts of the protein molecule is possible.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brewer J. M., Weber G. The effect of magnesium on some physical properties of yeast enolase. J Biol Chem. 1966 Jun 10;241(11):2550–2557. [PubMed] [Google Scholar]
- Chen R. F., Vurek G. G., Alexander N. Fluorescence decay times: proteins, coenzymes, and other compounds in water. Science. 1967 May 19;156(3777):949–951. doi: 10.1126/science.156.3777.949. [DOI] [PubMed] [Google Scholar]
- Delpierre G. R., Fruton J. S. Specific inactivation of pepsin by a diazo ketone. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1817–1822. doi: 10.1073/pnas.56.6.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Edelhoch H., Steiner R. F. Properties of thyroglobulin. XII. Comparison of the configurational states of reduced and unreduced thyroglobulin. Biopolymers. 1966 Oct-Nov;4(9):999–1014. doi: 10.1002/bip.1966.360040905. [DOI] [PubMed] [Google Scholar]
- FRATTALI V., STEINER R. F., EDELHOCH H. NATIVE AND UNFOLDED STATES OF PEPSINOGEN. I. THE MOLECULAR CONFORMATION IN WATER AND IN UREA. J Biol Chem. 1965 Jan;240:112–121. [PubMed] [Google Scholar]
- JOHNSON P., RICHARDS E. G. The study of legumin by depolarization of fluorescence and other physicochemical methods. Arch Biochem Biophys. 1962 May;97:260–276. doi: 10.1016/0003-9861(62)90078-4. [DOI] [PubMed] [Google Scholar]
- Johnson P., Mihalyi E. Physicochemical studies of bovine fibrinogen. II. Depolarization of fluorescence studies. Biochim Biophys Acta. 1965 Jul 22;102(2):476–486. doi: 10.1016/0926-6585(65)90138-x. [DOI] [PubMed] [Google Scholar]
- McCallum G. J., Woodward R. N. Cadmium-109 in New Zealand rainwater. Nature. 1966 Jan 1;209(5018):69–70. doi: 10.1038/209069b0. [DOI] [PubMed] [Google Scholar]
- Rajagopalan T. G., Moore S., Stein W. H. Pepsin from pepsinogen. Preparation and properties. J Biol Chem. 1966 Nov 10;241(21):4940–4950. [PubMed] [Google Scholar]
- Schlamowitz M., Shaw A., Jackson W. T. The nature of the binding of inhibitors to pepsin and the kinetics of inhibited peptic hydrolysis of N-acetyl-L-phenylalanyl-L-tyrosine. J Biol Chem. 1968 May 25;243(10):2821–2828. [PubMed] [Google Scholar]
- TEALE F. W. The ultraviolet fluorescence of proteins in neutral solution. Biochem J. 1960 Aug;76:381–388. doi: 10.1042/bj0760381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEALE F. W., WEBER G. Ultraviolet fluorescence of the aromatic amino acids. Biochem J. 1957 Mar;65(3):476–482. doi: 10.1042/bj0650476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazina A. A., Lednev V. V., Lemazhikhin B. K. Molekuliarney kharakteristiki pepsina v rastvore i priroda sil, stabiliziruiushchikh kompaktnuiu globulu pepsina. Biokhimiia. 1966 Jul-Aug;31(4):720–725. [PubMed] [Google Scholar]
- Vazina A. A., Lemazhikhin B. K. Opredelenie strukturnykh parametrov molekuly pepsinogena. Biofizika. 1966;11(5):779–784. [PubMed] [Google Scholar]
- WEBER G. Fluorescence-polarization spectrum and electronic-energy transfer in proteins. Biochem J. 1960 May;75:345–352. doi: 10.1042/bj0750345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBER G. Rotational Brownian motion and polarization of the fluorescence of solutions. Adv Protein Chem. 1953;8:415–459. doi: 10.1016/s0065-3233(08)60096-0. [DOI] [PubMed] [Google Scholar]
- Wahl P. Measure de la décroissange de la fluorescence polarisée de la gamma-globuline-1-sulfonyl-5-diméthylaminonaphthalène. Biochim Biophys Acta. 1969 Feb 4;175(1):55–64. [PubMed] [Google Scholar]
- Wahl P., Weber G. Fluorescence depolarization of rabbit gamma globulin conjugates. J Mol Biol. 1967 Dec 14;30(2):371–382. doi: 10.1016/s0022-2836(67)80045-7. [DOI] [PubMed] [Google Scholar]
- Weber G., Anderson S. R. The effects of energy transfer and rotational diffusion upon the fluorescence polarization of macromolecules. Biochemistry. 1969 Jan;8(1):361–371. doi: 10.1021/bi00829a050. [DOI] [PubMed] [Google Scholar]
- Winkler M. H. Fluorescence depolarization. A study of the influence of varying excitation wavelength and solution concentration. Biochim Biophys Acta. 1965 Jul 22;102(2):459–466. doi: 10.1016/0926-6585(65)90136-6. [DOI] [PubMed] [Google Scholar]


