Abstract
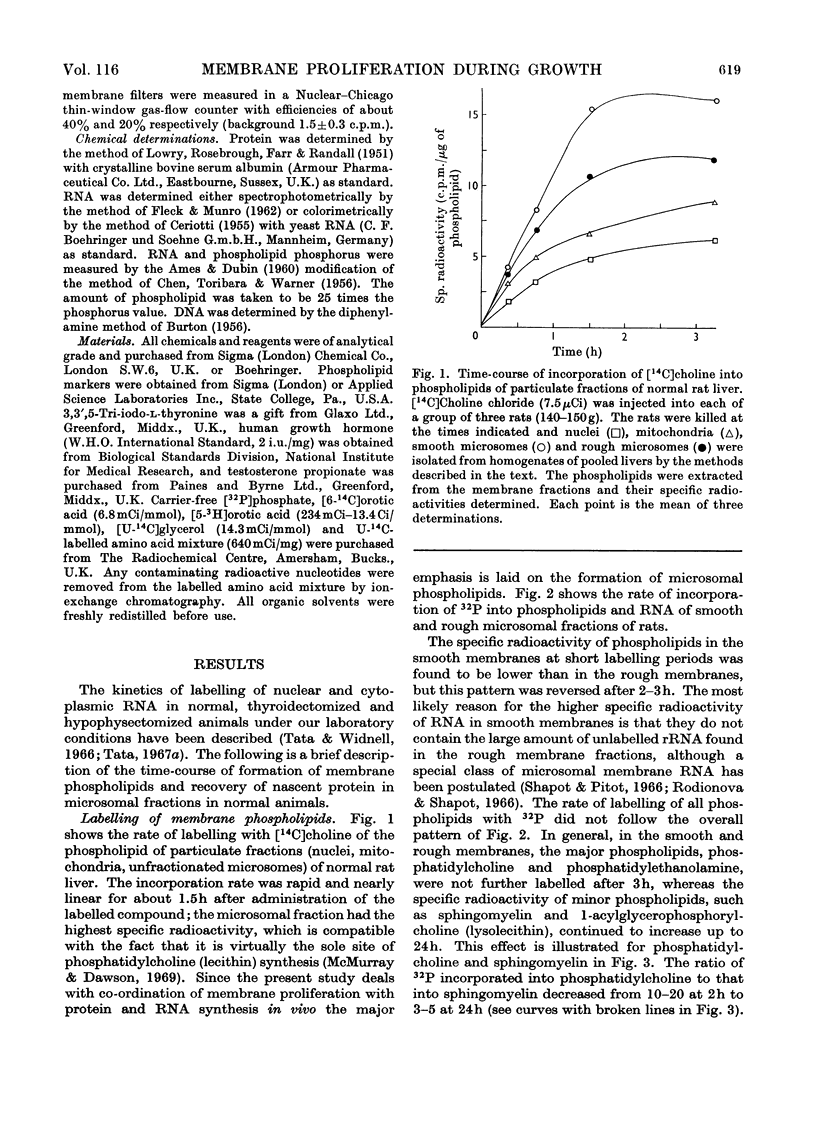
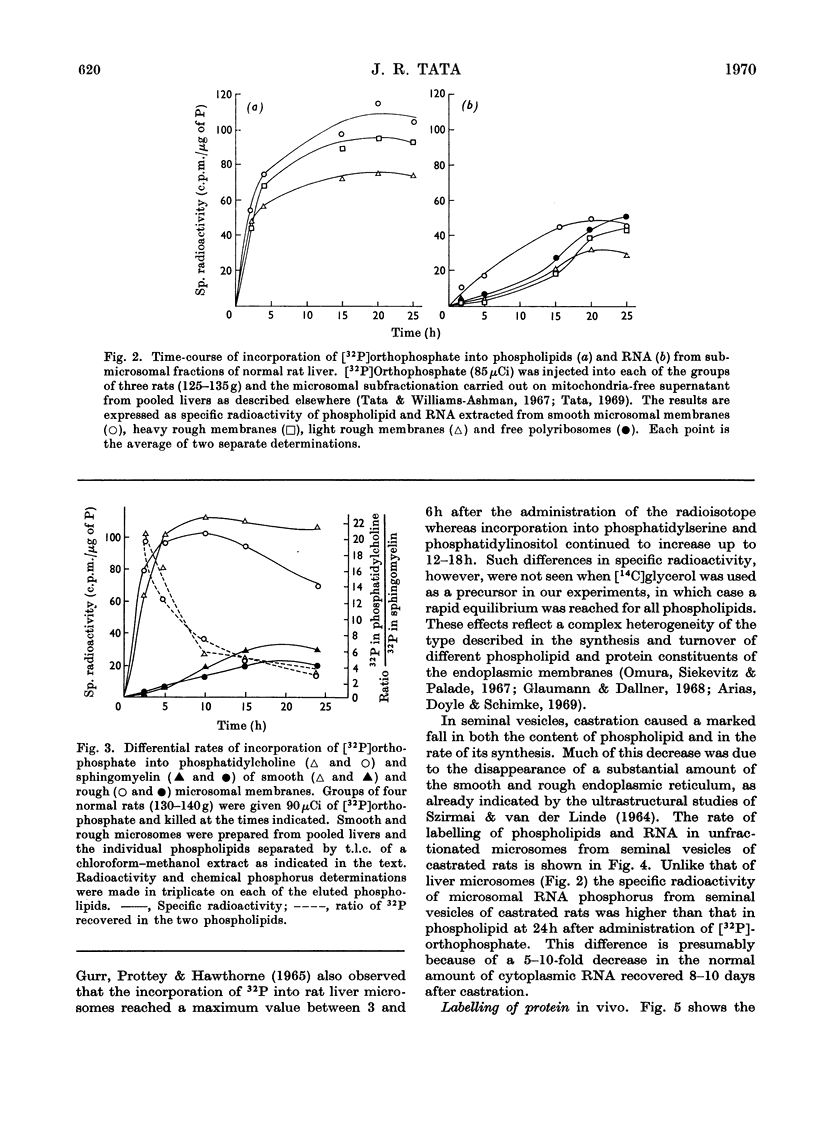
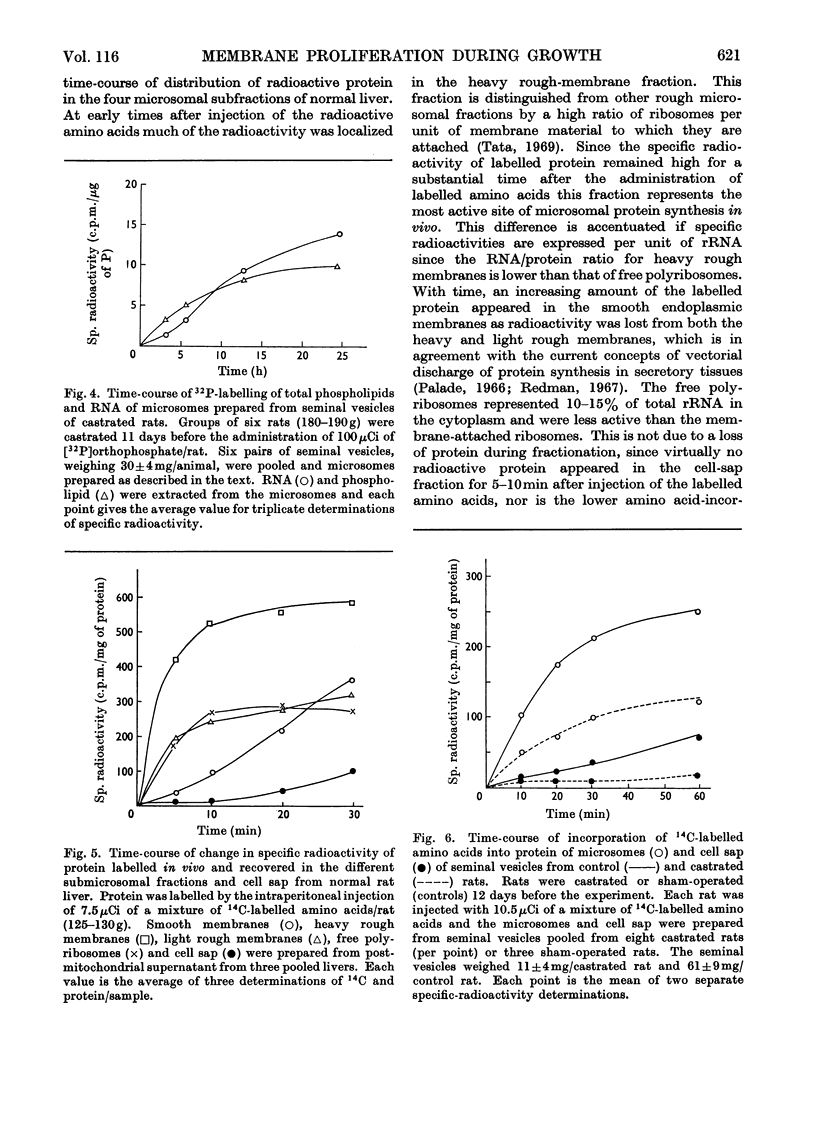
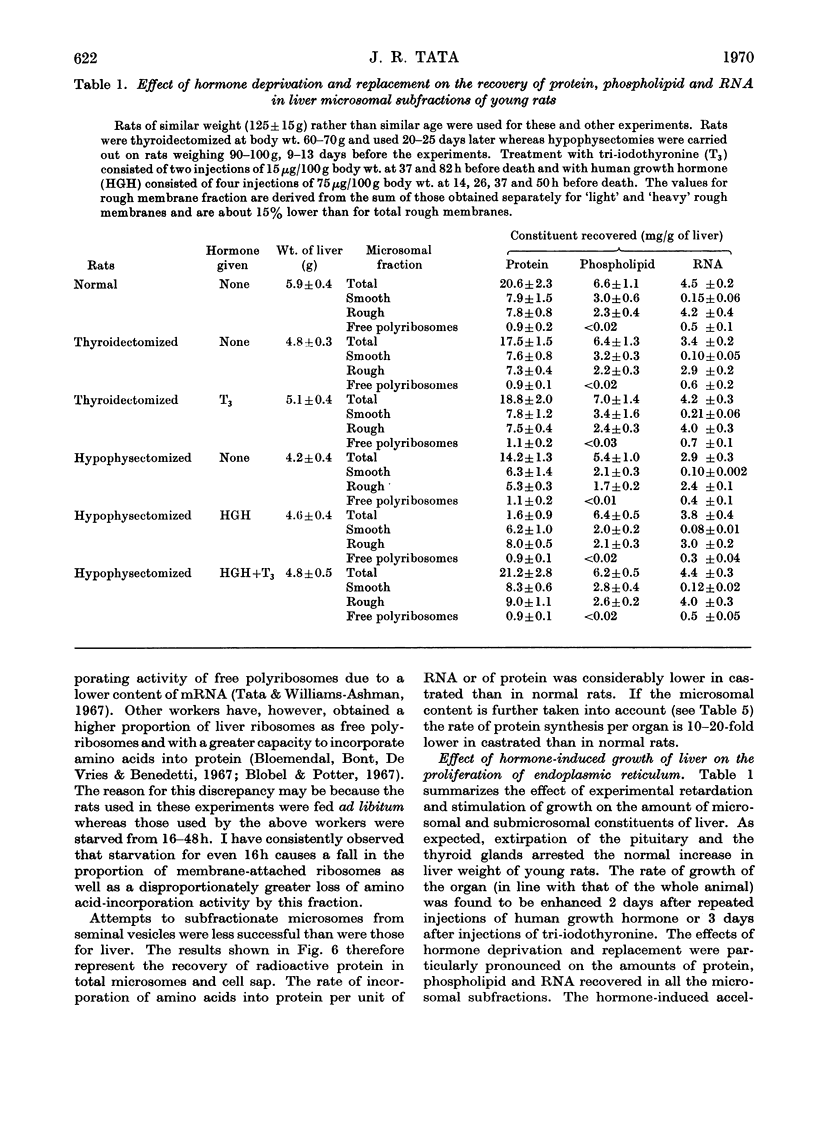
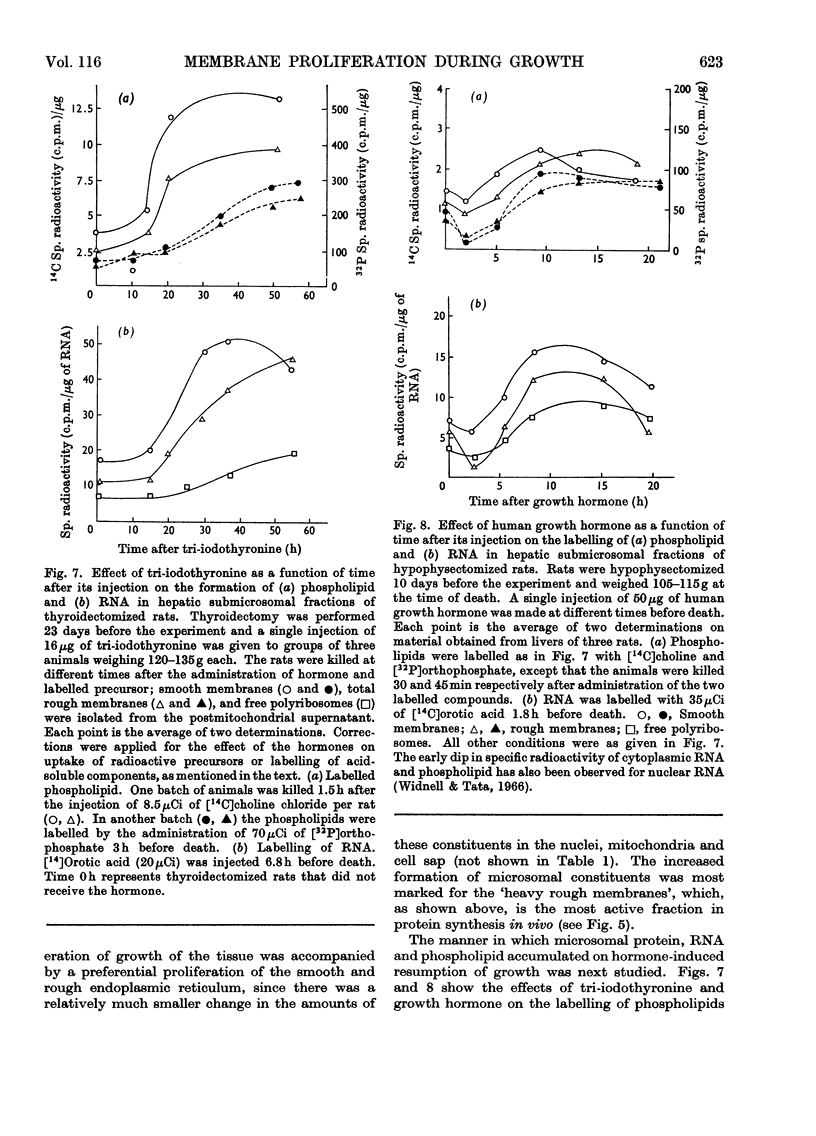
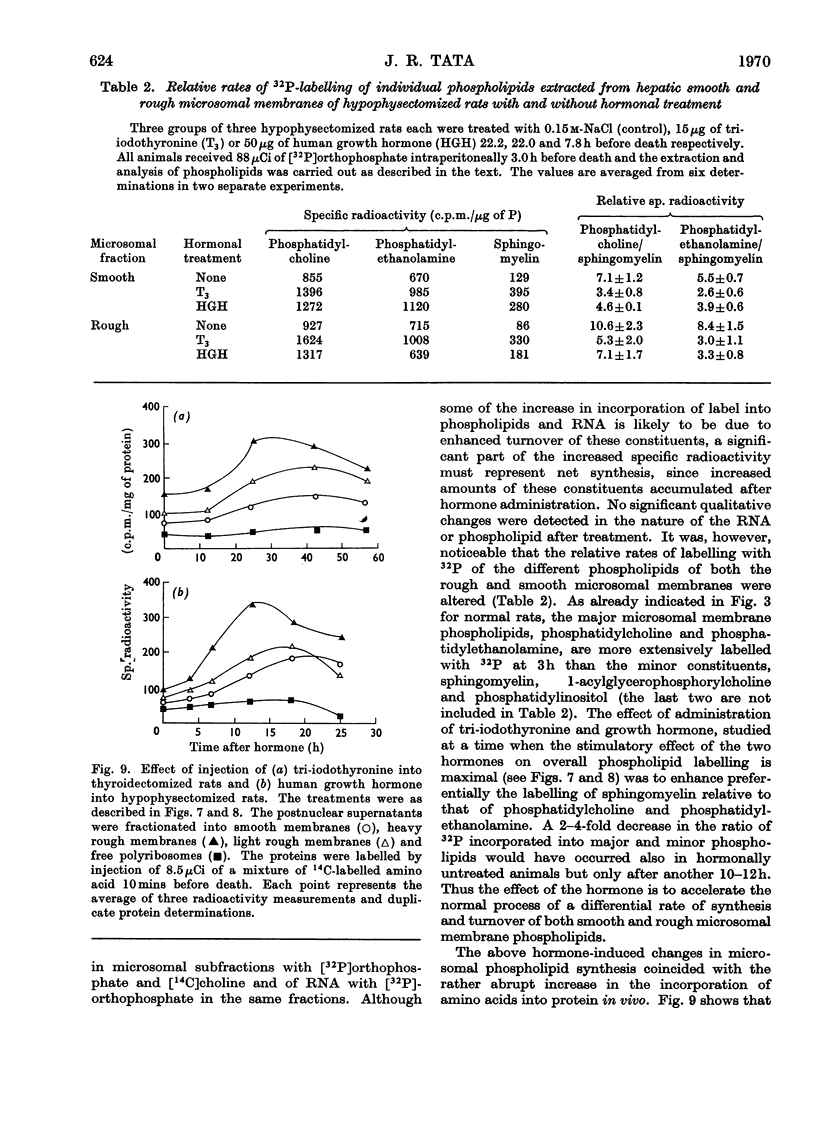
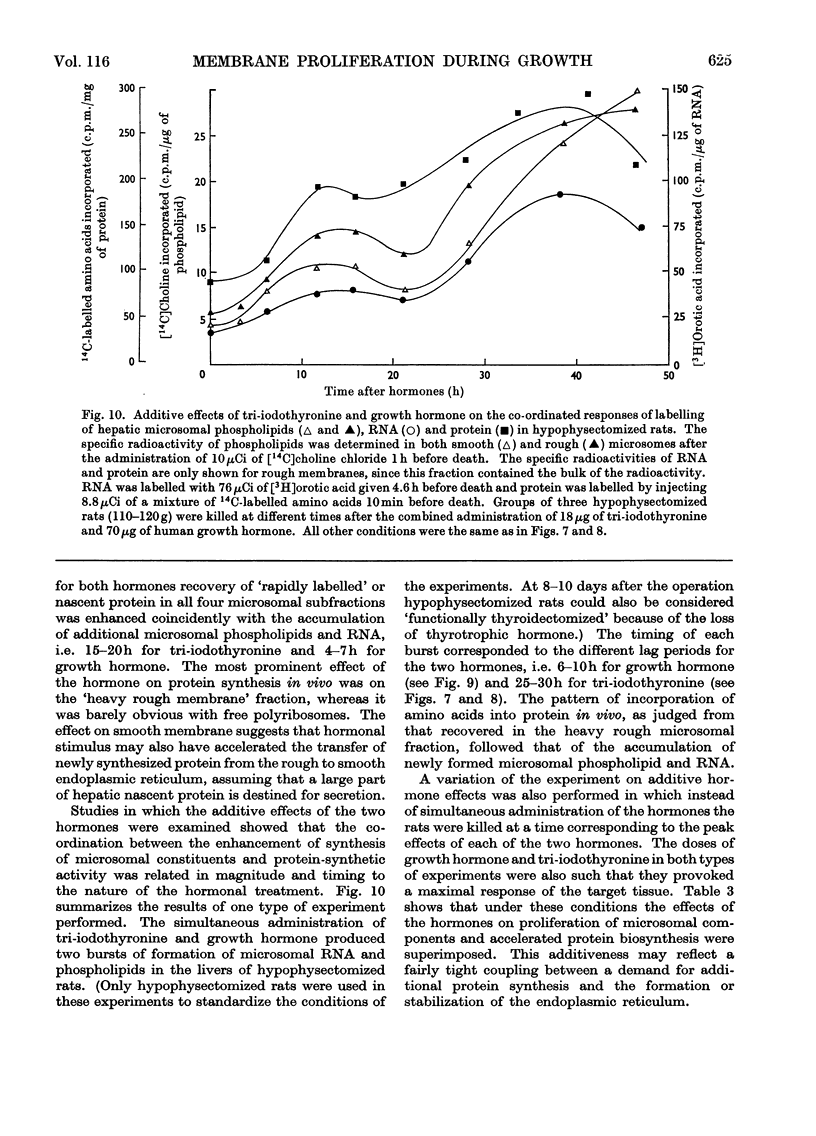
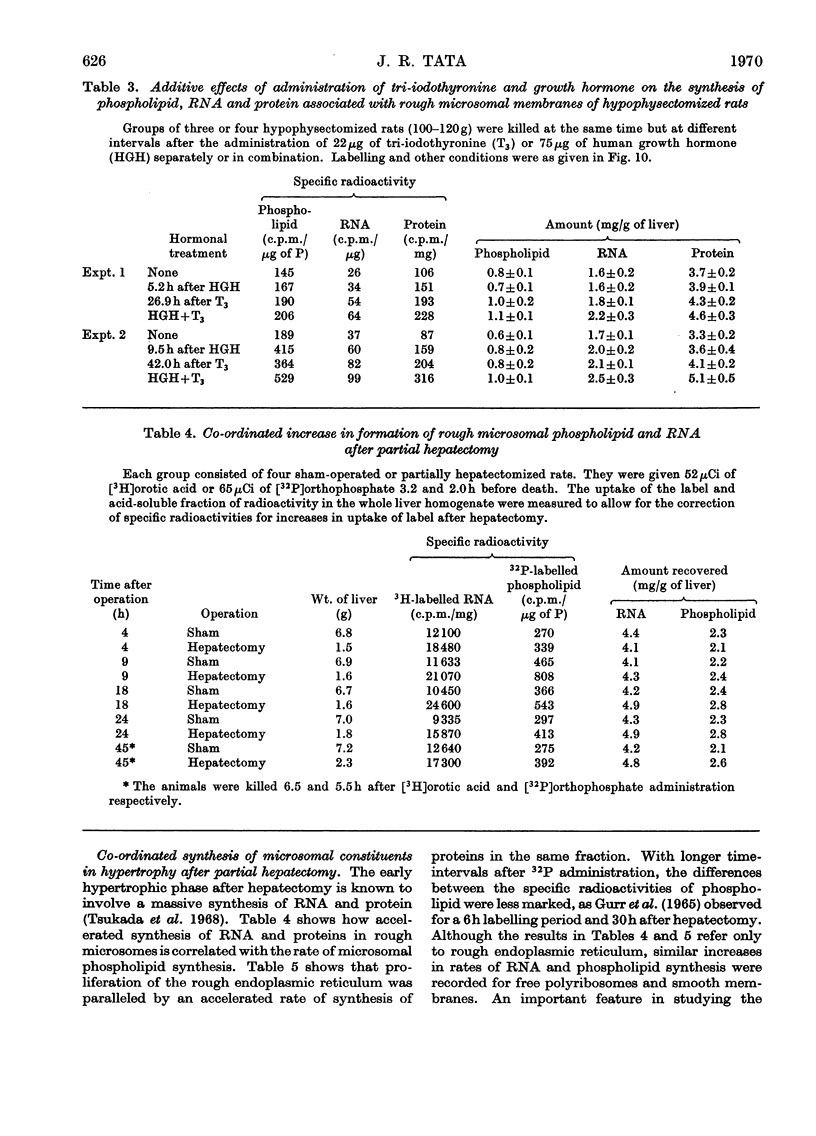
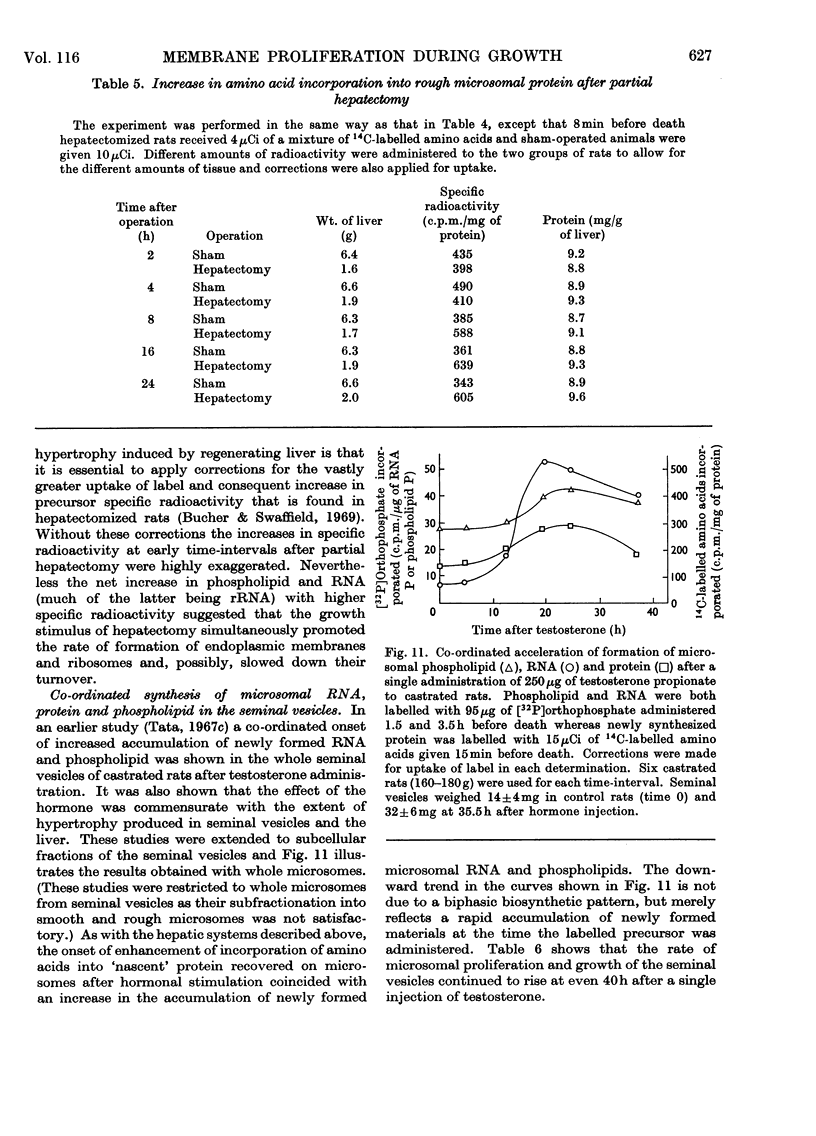
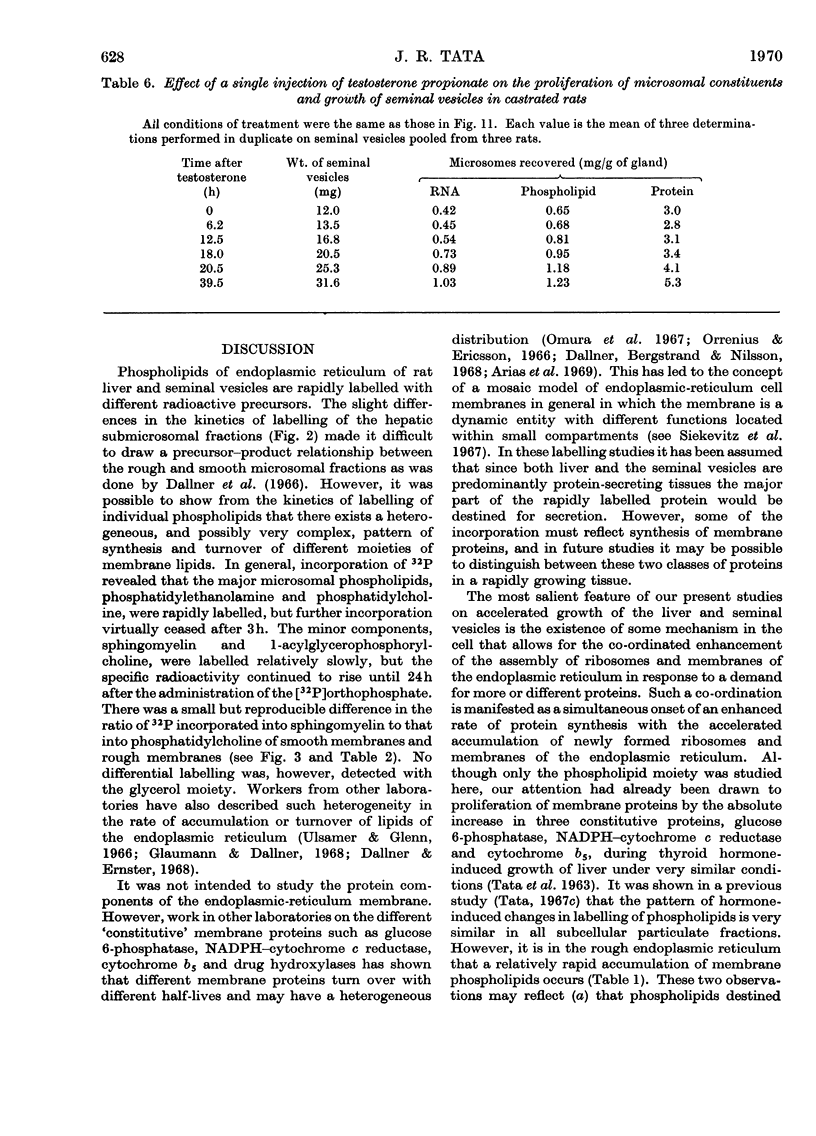
1. The rate of synthesis of membrane phospholipid was studied in rat liver and seminal vesicles by following the incorporation of [32P]orthophosphate, [14C]choline and [14C]glycerol. Particular emphasis was laid on the endoplasmic reticulum, which was fractionated into smooth microsomal membranes, heavy rough membranes, light rough membranes and free polyribosomes. 2. Phospholipid labelling patterns suggested a heterogeneity in the synthesis and turnover of the different lipid moieties of smooth and rough endoplasmic membranes. The major phospholipids, phosphatidylcholine and phosphatidylethanolamine, were labelled relatively rapidly with 32P over a short period of time whereas incorporation of radioisotope into the minor phospholipids, sphingomyelin, lysolecithin and phosphatidylinositol proceeded slowly but over a longer period of time. 3. The incorporation of orotic acid into RNA and labelled amino acids into protein of the four submicrosomal fractions was also studied. 4. Rapid growth of the liver was induced by the administration of growth hormone and tri-iodothyronine to hypophysectomized and thyroidectomized rats and by partial hepatectomy. Growth of seminal vesicles of castrated rats was stimulated with testosterone propionate. 5. The rate of labelling of membrane phospholipids was enhanced in all major subcellular particulate fractions (nuclear, mitochondrial and microsomal) during induced growth. However, it was in the rough endoplasmic reticulum that the accumulation of phospholipids, RNA and protein was most marked. The effect of hormone administration was also to accelerate preferentially the labelling with 32P of sphingomyelin relative to that of phosphatidylcholine or phosphatidylethanolamine. 6. Time-course analyses showed that, in all four growth systems studied, the enhancement of the rate of membrane phospholipid synthesis coincided with the rather abrupt increase in the synthesis of RNA and protein of the rough endoplasmic reticulum. Growth hormone and tri-iodothyronine administered to hypophysectomized rats had additive effects in all the biosynthetic processes. The latent period of action of each hormone was maintained so that two waves of proliferation of endoplasmic reticulum occurred if the hormones were administered simultaneously. 7. It is concluded that there is some mechanism in the cell that tightly co-ordinates the formation of membranes, especially those of the endoplasmic reticulum, when an increased demand is made for protein synthesis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AIZAWA Y., MUELLER G. C. The effect in vivo and in vitro of estrogens on lipid synthesis in the rat uterus. J Biol Chem. 1961 Feb;236:381–386. [PubMed] [Google Scholar]
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Andrews T. M., Tata J. R. Difference in vectorial release of nascent protein from membrane-bound ribosomes of secretory and non-secretory tissues. Biochem Biophys Res Commun. 1968 Sep 30;32(6):1050–1056. doi: 10.1016/0006-291x(68)90136-8. [DOI] [PubMed] [Google Scholar]
- Arias I. M., Doyle D., Schimke R. T. Studies on the synthesis and degradation of proteins of the endoplasmic reticulum of rat liver. J Biol Chem. 1969 Jun 25;244(12):3303–3315. [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Studies on free and membrane-bound ribosomes in rat liver. I. Distribution as related to total cellular RNA. J Mol Biol. 1967 Jun 14;26(2):279–292. doi: 10.1016/0022-2836(67)90297-5. [DOI] [PubMed] [Google Scholar]
- Bloemendal H., Bont W. S., de Vries M., Benedetti E. L. Isolation and properties of polyribosomes and fragments of the endoplasmic reticulum from rat liver. Biochem J. 1967 Apr;103(1):177–182. doi: 10.1042/bj1030177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher N. L., Swaffield M. N. Ribonucleic acid synthesis in relation to precursor pools in regenerating rat liver. Biochim Biophys Acta. 1969 Feb 18;174(2):491–502. doi: 10.1016/0005-2787(69)90278-0. [DOI] [PubMed] [Google Scholar]
- CERIOTTI G. Determination of nucleic acids in animal tissues. J Biol Chem. 1955 May;214(1):59–70. [PubMed] [Google Scholar]
- Campbell P. N., Cooper C., Hicks M. Studies on the role of the morphological constituents of the microsome fraction from rat liver in protein synthesis. Biochem J. 1964 Aug;92(2):225–234. doi: 10.1042/bj0920225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell P. N., Lowe E., Serck-Hanssen G. Protein synthesis by microsomal particles from regenerating rat liver. Biochem J. 1967 Apr;103(1):280–288. doi: 10.1042/bj1030280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallner G., Bergstrand A., Nilsson R. Heterogeneity of rough-surfaced liver microsomal membranes of adult, phenobarbital-treated, and newborn rats. J Cell Biol. 1968 Aug;38(2):257–276. doi: 10.1083/jcb.38.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallner G., Ernster L. Subfractionation and composition of microsomal membranes: a review. J Histochem Cytochem. 1968 Oct;16(10):611–632. doi: 10.1177/16.10.611. [DOI] [PubMed] [Google Scholar]
- Dallner G., Siekevitz P., Palade G. E. Biogenesis of endoplasmic reticulum membranes. I. Structural and chemical differentiation in developing rat hepatocyte. J Cell Biol. 1966 Jul;30(1):73–96. doi: 10.1083/jcb.30.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doeg K. A. Control of mitochondrial lipid biosynthesis by testosterone in male sex accessory gland tissue and liver of castrate rats. Endocrinology. 1968 Mar;82(3):535–539. doi: 10.1210/endo-82-3-535. [DOI] [PubMed] [Google Scholar]
- FLECK A., MUNRO H. N. The precision of ultraviolet absorption measurements in the Schmidt-Thannhauser procedure for nucleic acid estimation. Biochim Biophys Acta. 1962 May 14;55:571–583. doi: 10.1016/0006-3002(62)90836-3. [DOI] [PubMed] [Google Scholar]
- Fisher D. B., Mueller G. C. An early alteration in the phospholipid metabolism of lymphocytes by phytohemagglutinin. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1396–1402. doi: 10.1073/pnas.60.4.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii T., Villee C. RNA metabolism in seminal vesicle and prostate of immature rats following castration and androgen replacement. Acta Endocrinol (Copenh) 1969 Mar;60(3):527–536. doi: 10.1530/acta.0.0600527. [DOI] [PubMed] [Google Scholar]
- Glaumann H., Dallner G. Lipid composition and turnover of rough and smooth microsomal membranes in rat liver. J Lipid Res. 1968 Nov;9(6):720–729. [PubMed] [Google Scholar]
- Gurr M. I., Prottey C., Hawthorne J. N. The phospholipids of liver-cell fractions. II. Incorporation of [32P]orthophosphate in vivo in normal and regenerating rat liver. Biochim Biophys Acta. 1965 Oct 4;106(2):357–370. [PubMed] [Google Scholar]
- HENSHAW E. C., BOJARSKI T. B., HIATT H. H. PROTEIN SYNTHESIS BY FREE AND BOUND RAT LIVER RIBOSOMES IN VIVO AND IN VITRO. J Mol Biol. 1963 Aug;7:122–129. doi: 10.1016/s0022-2836(63)80041-8. [DOI] [PubMed] [Google Scholar]
- Jos J., Frezal J., Rey J., Lamy M. Histochemical localization of intestinal disaccharidases: application to peroral biopsy specimens. Nature. 1967 Feb 4;213(5075):516–518. doi: 10.1038/213516a0. [DOI] [PubMed] [Google Scholar]
- Kerkof P. R., Tata J. R. Simultaneous acceleration in vivo of the formation of thyroid ribonucleic acid, phospholipid and iodoprotein by thyroid-stimulating hormone. Biochem Biophys Res Commun. 1967 Jul 10;28(1):111–116. doi: 10.1016/0006-291x(67)90414-7. [DOI] [PubMed] [Google Scholar]
- Kerkof P. R., Tata J. R. The subcellular distribution of 32P-labelled phospholipids, 32P-labelled ribonucleic acid and 125I-labelled odoprotein in pig thyroid slices. Effect in vitro of thyrotrophic hormone and dibutyryl-3',5'-(cyclic)-adeosine onophosphate. Biochem J. 1969 May;112(5):729–739. doi: 10.1042/bj1120729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leduc E. H., Avrameas S., Bouteille M. Ultrastructural localization of antibody in differentiating plasma cells. J Exp Med. 1968 Jan 1;127(1):109–118. doi: 10.1084/jem.127.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainwaring W. I. The effect of age on protein synthesis in mouse liver. Biochem J. 1969 Aug;113(5):869–878. doi: 10.1042/bj1130869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray W. C., Dawson R. M. Phospholipid exchange reactions within the liver cell. Biochem J. 1969 Mar;112(1):91–108. doi: 10.1042/bj1120091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls T. J., Follett B. K., Evennett P. J. The effects of oestrogens and other steroid hormones on the ultrastructure of the liver of Xenopus laevis Daudin. Z Zellforsch Mikrosk Anat. 1968;90(1):19–27. doi: 10.1007/BF00496699. [DOI] [PubMed] [Google Scholar]
- Omura T., Siekevitz P., Palade G. E. Turnover of constituents of the endoplasmic reticulum membranes of rat hepatocytes. J Biol Chem. 1967 May 25;242(10):2389–2396. [PubMed] [Google Scholar]
- Orrenius S., Ericsson J. L. Enzyme-membrane relationship in phenobarbital induction of synthesis of drug-metabolizing enzyme system and proliferation of endoplasmic membranes. J Cell Biol. 1966 Feb;28(2):181–198. doi: 10.1083/jcb.28.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade G. E. Structure and function at the cellular level. JAMA. 1966 Nov 21;198(8):815–825. [PubMed] [Google Scholar]
- Pollak J. K., Ward D. B. Changes in the chemical composition and the enzymic activities of hepatic microsomes of the chick embryo during development. Biochem J. 1967 Jun;103(3):730–738. doi: 10.1042/bj1030730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman C. M. Studies on the transfer of incomplete polypeptide chains across rat liver microsomal membranes in vitro. J Biol Chem. 1967 Feb 25;242(4):761–768. [PubMed] [Google Scholar]
- Rodionova N. P., Shapot V. S. Ribonucleic acids of the endoplasmic reticulum of animal cells. Biochim Biophys Acta. 1966 Oct 24;129(1):206–209. doi: 10.1016/0005-2787(66)90024-4. [DOI] [PubMed] [Google Scholar]
- SZIRMAI J. A., VAN DER LINDE P. C. EFFECT OF CASTRATION ON THE ENDOPLASMIC RETICULUM OF THE SEMINAL VESICLE AND OTHER TARGET EPITHELIA IN THE RAT. J Ultrastruct Res. 1965 Apr;12:380–395. doi: 10.1016/s0022-5320(65)80106-x. [DOI] [PubMed] [Google Scholar]
- Shapot V., Pitot H. C. Isolation and fractionation of ribonucleic acid from the smooth endoplasmic reticulum of rat liver. Biochim Biophys Acta. 1966 Apr 18;119(1):37–45. doi: 10.1016/0005-2787(66)90035-9. [DOI] [PubMed] [Google Scholar]
- TATA J. R., ERNSTER L., LINDBERG O., ARRHENIUS E., PEDERSEN S., HEDMAN R. The action of thyroid hormones at the cell level. Biochem J. 1963 Mar;86:408–428. doi: 10.1042/bj0860408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata J. R. Early metamorphic competence of Xenopus larvae. Dev Biol. 1968 Nov;18(5):415–440. doi: 10.1016/0012-1606(68)90050-x. [DOI] [PubMed] [Google Scholar]
- Tata J. R. Hormonal regulation of growth and protein synthesis. Nature. 1968 Jul 27;219(5152):331–337. doi: 10.1038/219331a0. [DOI] [PubMed] [Google Scholar]
- Tata J. R. The formation and distribution of ribosomes during hormone-induced growth and development. Biochem J. 1967 Jul;104(1):1–16. doi: 10.1042/bj1040001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata J. R. The formation, distribution and function of ribosomes and microsomal membranes during induced amphibian metamorphosis. Biochem J. 1967 Nov;105(2):783–801. doi: 10.1042/bj1050783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata J. R., Williams-Ashman H. G. Effects of growth hormone and tri-iodothyronine on amino acid incorporation by microsomal subfractions from rat liver. Eur J Biochem. 1967 Oct;2(3):366–374. doi: 10.1111/j.1432-1033.1967.tb00147.x. [DOI] [PubMed] [Google Scholar]
- Teng C. S., Hamilton T. H. Regulation of polyribosome formation and protein synthesis in the uterus. Effect of ovariectomy and administration of oestradiol-17beta on the amino acid-incorporation activity in vitro and the cytoplasmic concentration in vivo of polyribosomal preparation. Biochem J. 1967 Dec;105(3):1101–1109. doi: 10.1042/bj1051101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada K., Moriyama T., Umeda T., Lieberman I. Relationship between the ribosomal alteration after partial hepatectomy and the increase in liver protein synthesis in vivo. J Biol Chem. 1968 Mar 25;243(6):1160–1165. [PubMed] [Google Scholar]
- Ulsamer A. G., Glenn J. L. The incorporation of radioactive phosphorus into individual liver phosphatides of rats. Biochim Biophys Acta. 1966 Dec 7;125(3):525–533. doi: 10.1016/0005-2760(66)90040-3. [DOI] [PubMed] [Google Scholar]
- Widnell C. C., Tata J. R. A procedure for the isolation of enzymically active rat-liver nuclei. Biochem J. 1964 Aug;92(2):313–317. doi: 10.1042/bj0920313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widnell C. C., Tata J. R. Additive effects of thyroid hormone, growth hormone and testosterone on deoxyribonucleic acid-dependent ribonucleic acid polymerase in rat-liver nuclei. Biochem J. 1966 Feb;98(2):621–629. doi: 10.1042/bj0980621. [DOI] [PMC free article] [PubMed] [Google Scholar]


