Abstract
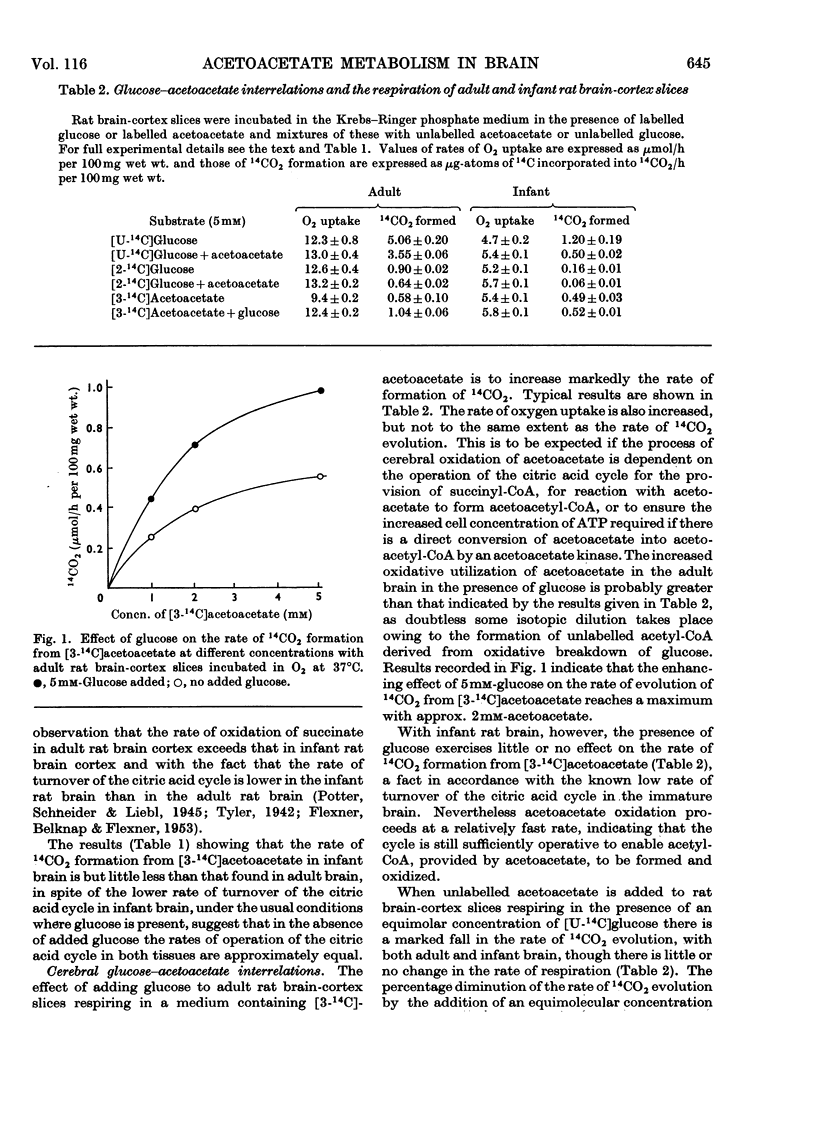
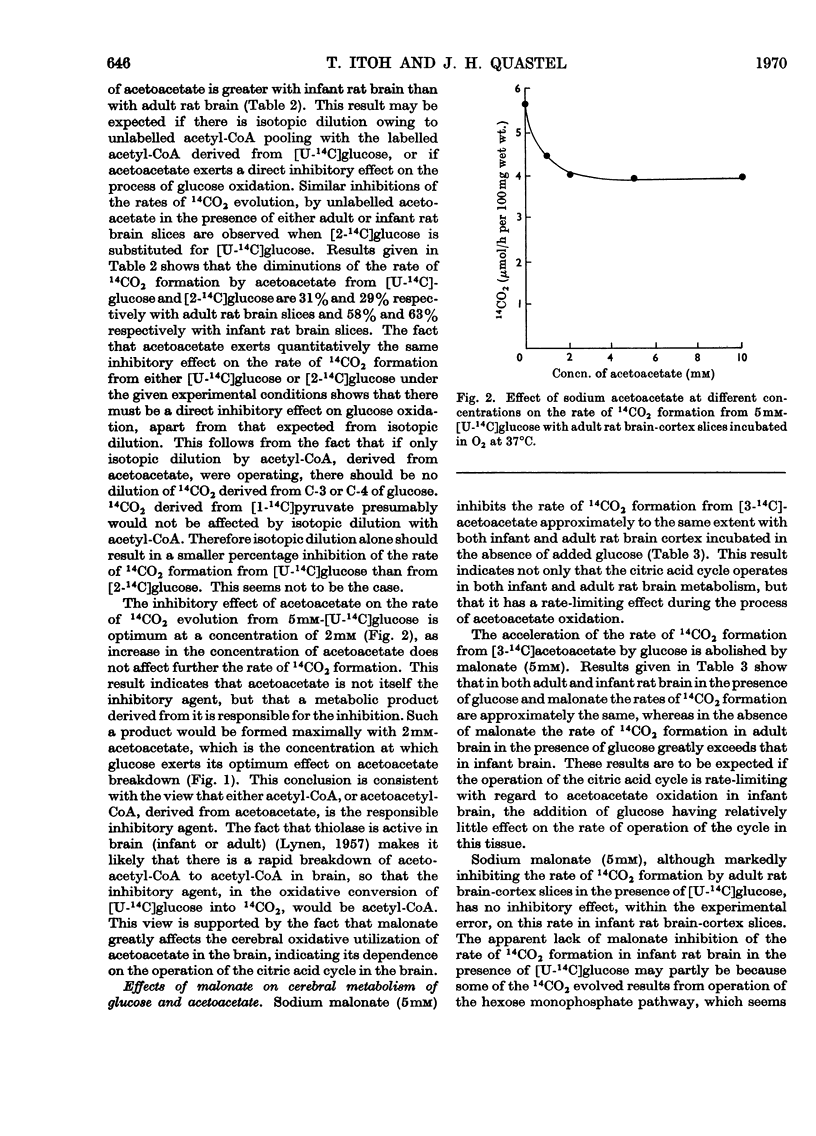
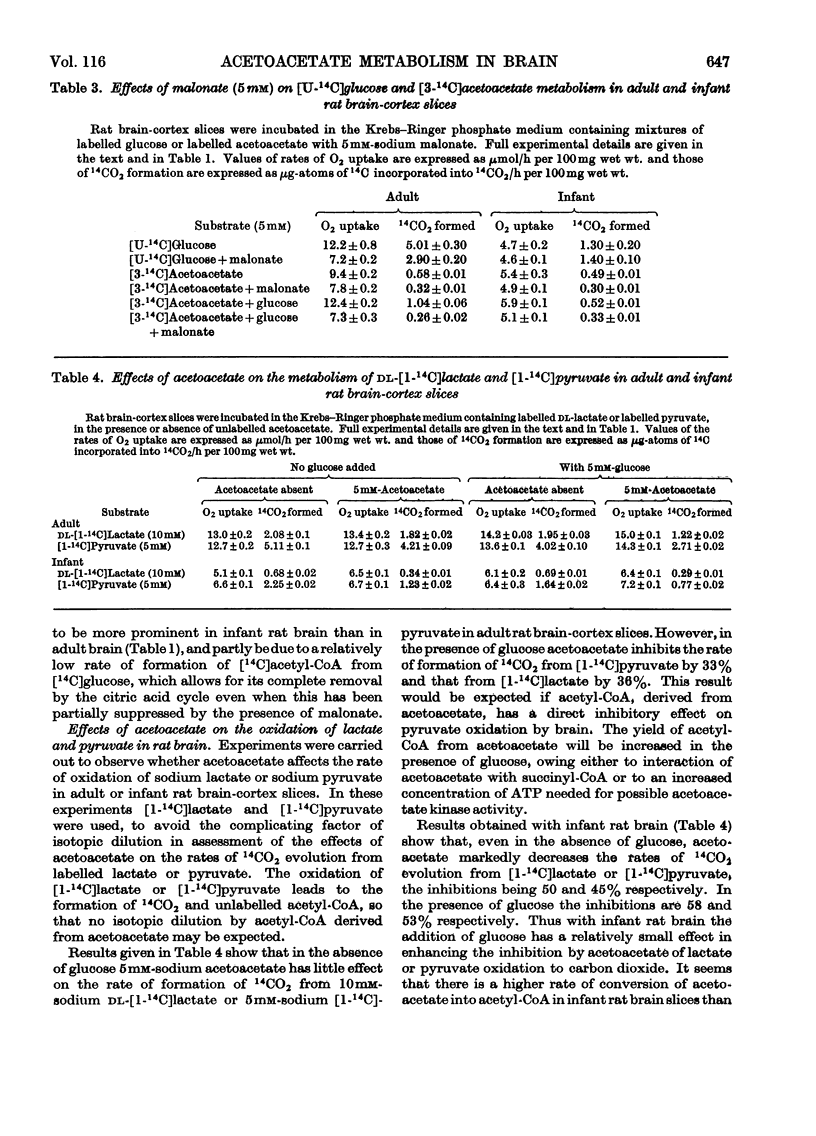
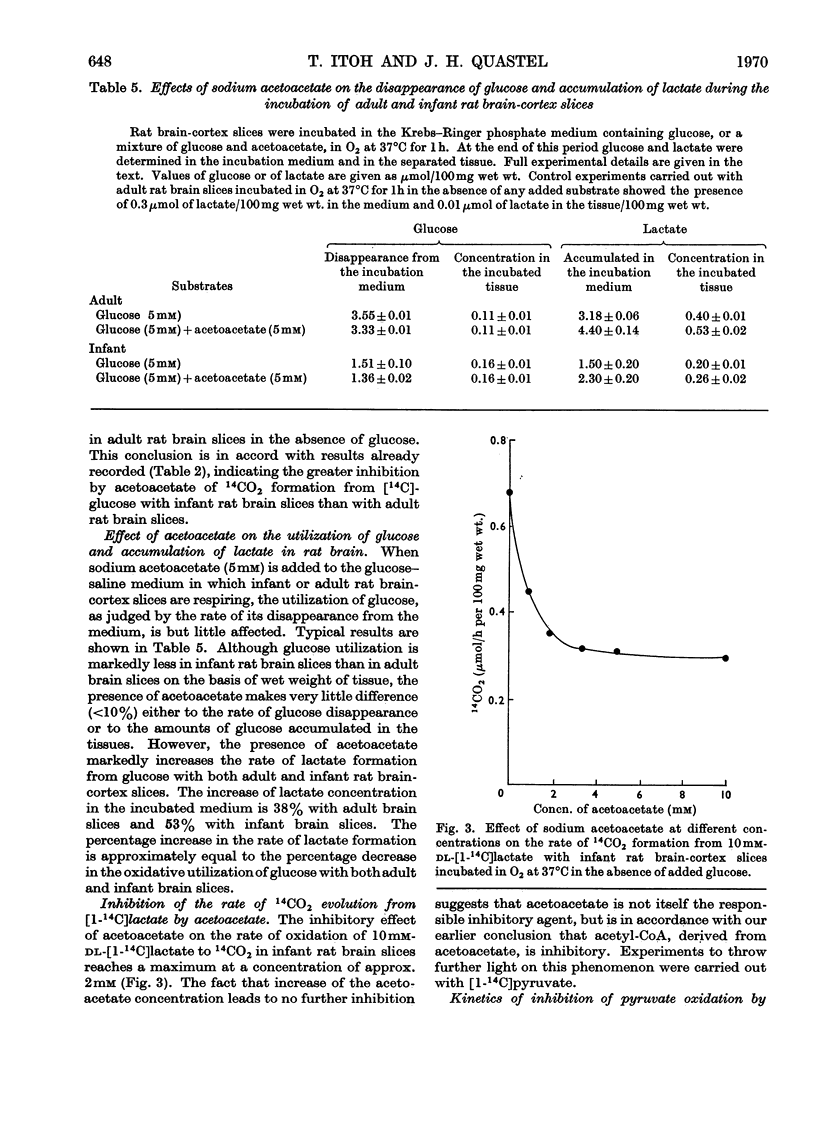
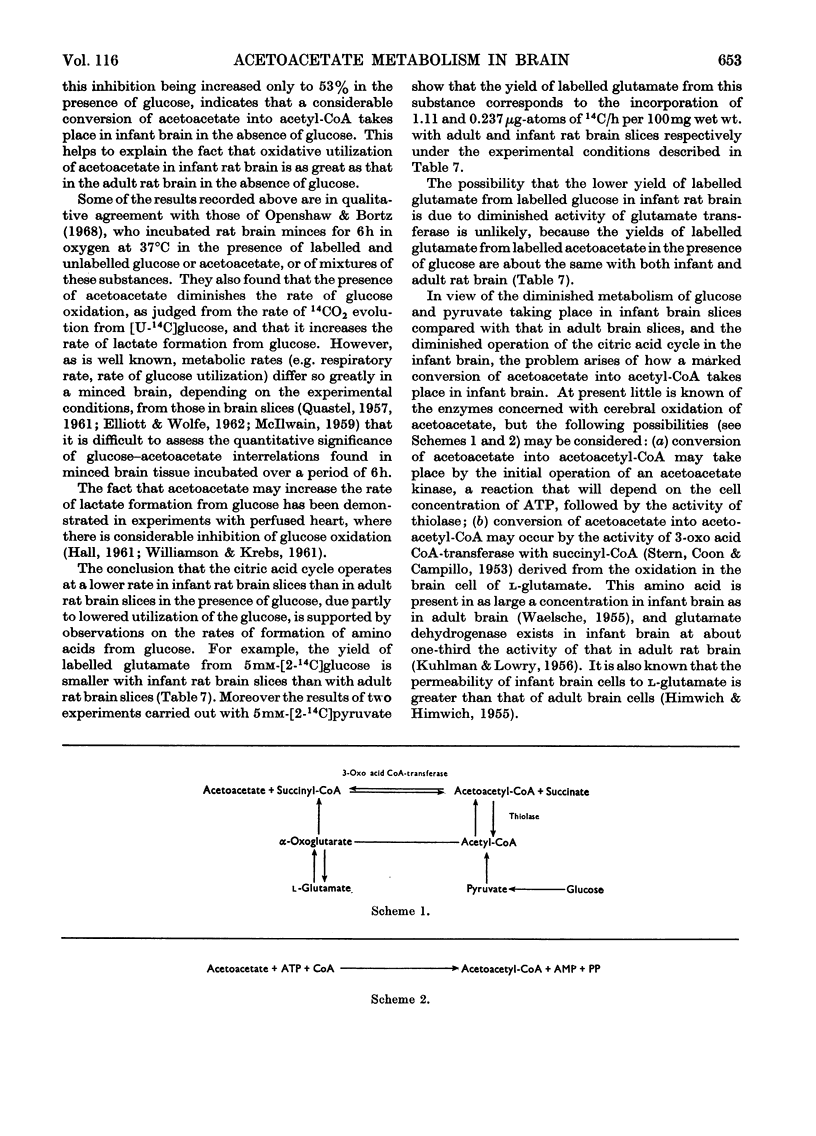
1. Acetoacetate or dl-β-hydroxybutyrate increases the rate of oxygen consumption to a smaller extent than that brought about by glucose or pyruvate in adult rat brain-cortex slices but to the same extent as that in infant rat brain-cortex slices. 2. The rate of 14CO2 evolution from [1-14C]glucose considerably exceeds that from [6-14C]glucose in respiring infant rat brain-cortex slices, in contrast with adult brain-cortex slices, suggesting that the hexose monophosphate shunt operates at a greater rate in the infant rat brain than in the adult rat brain. 3. The rate of 14CO2 evolution from [3-14C]acetoacetate or dl-β-hydroxy[3-14C]butyrate, in the absence of glucose, is the same in infant rat brain slices as in adult rat brain slices. It exceeds that from [2-14C]glucose in infant rat brain but is less than that from [2-14C]glucose in adult rat brain. 4. Acetoacetate is oxidized in the brain through the operation of the citric acid cycle, as shown by the accelerating effect of glucose on acetoacetate oxidation in adult brain slices, by the inhibitory effects of malonate in both infant and adult brain slices and by its conversion into glutamate and related amino acids in both tissues. 5. Acetoacetate does not affect glucose utilization in adult or infant brain slices. It inhibits the rate of 14CO2 formation from [2-14C]glucose or [U-14C]-glucose the effect not being wholly due to isotopic dilution. 6. Acetoacetate inhibits non-competitively the oxidation of [1-14C]pyruvate, the effect being attributed to competition between acetyl-CoA and CoA for the pyruvate-oxidation system. 7. Acetoacetate increases the rate of aerobic formation of lactate from glucose with both adult and infant rat brain slices. 8. The presence of 0.1mm-2,4-dinitrophenol diminishes but does not abolish the rate of 14CO2 formation from [3-14C]acetoacetate in rat brain slices. This points to the participation of ATP in the process of oxidation of acetoacetate in infant or adult rat brain. 9. The presence of 5mm-d-glutamate inhibits the rate of 14CO2 formation from [3-14C]acetoacetate, in the presence or absence of glucose. 10. Labelled amino acids are formed from [3-14C]acetoacetate in both adult and infant rat brain-cortex slices, but the amounts are smaller than those found with [2-14C]glucose in adult rat brain and greater than those found with [2-14C]glucose in infant rat brain. 11. Acetoacetate is not as effective as glucose as a precursor of acetylcholine in adult rat brain but is as effective as glucose in infant rat brain slices. 12. Acetoacetate or β-hydroxybutyrate is a more potent source of acetyl-CoA than is glucose in infant rat brain slices but is less so in adult rat brain slices.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABADOM P. N., SCHOLEFIELD P. G. Amino acid transport in brain cortex slices. I. The relation between energy production and the glucose-dependent transport of glycine. Can J Biochem Physiol. 1962 Nov;40:1575–1590. [PubMed] [Google Scholar]
- ABADOM P. N., SCHOLEFIELD P. G. Amino acid transport in brain cortex slices. III. The utilization of energy for transport. Can J Biochem Physiol. 1962 Nov;40:1603–1618. [PubMed] [Google Scholar]
- Ames A., 3rd, Tsukada Y., Nesbett F. B. Intracellular Cl-, Na+, K+, Ca2+, Mg2+, and P in nervous tissue; response to glutamate and to changes in extracellular calcium. J Neurochem. 1967 Feb;14(2):145–159. doi: 10.1111/j.1471-4159.1967.tb05887.x. [DOI] [PubMed] [Google Scholar]
- Browning E. T., Schulman M. P. (14C) acetylcholine synthesis by cortex slices of rat brain. J Neurochem. 1968 Dec;15(12):1391–1405. doi: 10.1111/j.1471-4159.1968.tb05921.x. [DOI] [PubMed] [Google Scholar]
- CASE E. M., McILWAIN H. Respiration and phosphorylation in preparations from Mammalian Brain. Biochem J. 1951 Jan;48(1):1–11. doi: 10.1042/bj0480001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAUGHEY W. S., HELLERMAN L., SMILEY J. D. L-glutamic acid dehydrogenase; structural requirements for substrate competition; effect of thyroxine. J Biol Chem. 1957 Jan;224(1):591–607. [PubMed] [Google Scholar]
- DAVIS E. J., QUASTEL J. H. THE EFFECTS OF SHORT-CHAIN FATTY ACIDS AND STARVATION ON THE METABOLISM OF GLUCOSE AND LACTATE BY THE PERFUSED GUINEA PIG HEART. Can J Biochem. 1964 Nov;42:1605–1621. doi: 10.1139/o64-172. [DOI] [PubMed] [Google Scholar]
- DRAHOTA Z., HAHN P., MOUREK J., TROJANOVA M. THE EFFECT OF ACETOACETATE ON OXYGEN CONSUMPTION OF BRAIN SLICES FROM INFANT AND ADULT RATS. Physiol Bohemoslov. 1965;14:134–136. [PubMed] [Google Scholar]
- FLEXNER L. B., BELKNAP E. L., Jr, FLEXNER J. B. Biochemical and physiological differentiation during morphogenesis. XVI. Cytochrome oxidase, succinic dehydrogenase and succinoxidase in the developing cerebral cortex and liver of the fetal guinea pig. J Cell Physiol. 1953 Aug;42(1):151–161. doi: 10.1002/jcp.1030420110. [DOI] [PubMed] [Google Scholar]
- GOLDMAN D. S. Studies on the fatty acid oxidizing system of animal tissues. VII. The beta-ketoacyl coenzyme A cleavage enzyme. J Biol Chem. 1954 May;208(1):345–357. [PubMed] [Google Scholar]
- GONDA O., QUASTEL J. H. Effects of acetylsalicylate and 2, 4-dinitrophenol on metabolism and transport in rat brain cortex in vitro. Can J Biochem Physiol. 1963 Feb;41:435–454. [PubMed] [Google Scholar]
- Gonda O., Quastel J. H. Transport and metabolism of acetate in rat brain cortex in vitro. Biochem J. 1966 Jul;100(1):83–94. doi: 10.1042/bj1000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUHLMAN R. E., LOWRY O. H. Quantitative histochemical changes during the development of the rat cerebral cortex. J Neurochem. 1956 Dec;1(2):173–180. doi: 10.1111/j.1471-4159.1956.tb12070.x. [DOI] [PubMed] [Google Scholar]
- Krebs H. A., Hems R., Weidemann M. J., Speake R. N. The fate of isotopic carbon in kidney cortex synthesizing glucose from lactate. Biochem J. 1966 Oct;101(1):242–249. doi: 10.1042/bj1010242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A. Metabolism of amino-acids: The synthesis of glutamine from glutamic acid and ammonia, and the enzymic hydrolysis of glutamine in animal tissues. Biochem J. 1935 Aug;29(8):1951–1969. doi: 10.1042/bj0291951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann P. J., Tennenbaum M., Quastel J. H. On the mechanism of acetylcholine formation in brain in vitro. Biochem J. 1938 Feb;32(2):243–261. doi: 10.1042/bj0320243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes P. A., Felts J. M. Determination of 14C-labelled ketone bodies by liquid-scintillation counting. Biochem J. 1967 Jan;102(1):230–235. doi: 10.1042/bj1020230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourek J. Vztah kyseliny acetoctové a stárí k mnozství glyklogenu a k produkci kyseliny mléné v mozku krysy. Sb Lek. 1968 Jul;70(7):222–227. [PubMed] [Google Scholar]
- Openshaw H., Bortz W. M. Oxidation of glucose, acetoacetate, and palmitate in brain mince of normal and ketotic rats. Diabetes. 1968 Feb;17(2):90–95. doi: 10.2337/diab.17.2.90. [DOI] [PubMed] [Google Scholar]
- Owen O. E., Morgan A. P., Kemp H. G., Sullivan J. M., Herrera M. G., Cahill G. F., Jr Brain metabolism during fasting. J Clin Invest. 1967 Oct;46(10):1589–1595. doi: 10.1172/JCI105650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolleston F. S., Newsholme E. A. Effects of fatty acids, ketone bodies, lactate and pyruvate on glucose utilization by guinea-pig cerebral cortex slices. Biochem J. 1967 Aug;104(2):519–523. doi: 10.1042/bj1040519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STERN J. R., OCHOA S. Enzymatic synthesis of citric acid. I. Synthesis with soluble enzymes. J Biol Chem. 1951 Jul;191(1):161–172. [PubMed] [Google Scholar]
- TAKAGAKI G., HIRANO S., NAGATA Y. Some observations on the effect of D-glutamate on the glucose metabolism and the accumulation of potassium ions in brain cortex slices. J Neurochem. 1959 Jun;4(2):124–134. doi: 10.1111/j.1471-4159.1959.tb13181.x. [DOI] [PubMed] [Google Scholar]
- TSUKADA Y., NAGATA Y., HIRANO S., MATSUTANI T. Active transport of amino acid into cerebral cortex slices. J Neurochem. 1963 Apr;10:241–256. doi: 10.1111/j.1471-4159.1963.tb05040.x. [DOI] [PubMed] [Google Scholar]
- WEIL-MALHERBE H., GREEN R. H. Ammonia formation in brain. I. Studies on slices and suspensions. Biochem J. 1955 Oct;61(2):210–218. doi: 10.1042/bj0610210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMSON J. R., KREBS H. A. Acetoacetate as fuel of respiration in the perfused rat heart. Biochem J. 1961 Sep;80:540–547. doi: 10.1042/bj0800540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil-Malherbe H. Studies on brain metabolism: The metabolism of glutamic acid in brain. Biochem J. 1936 Apr;30(4):665–676. doi: 10.1042/bj0300665. [DOI] [PMC free article] [PubMed] [Google Scholar]


