Abstract
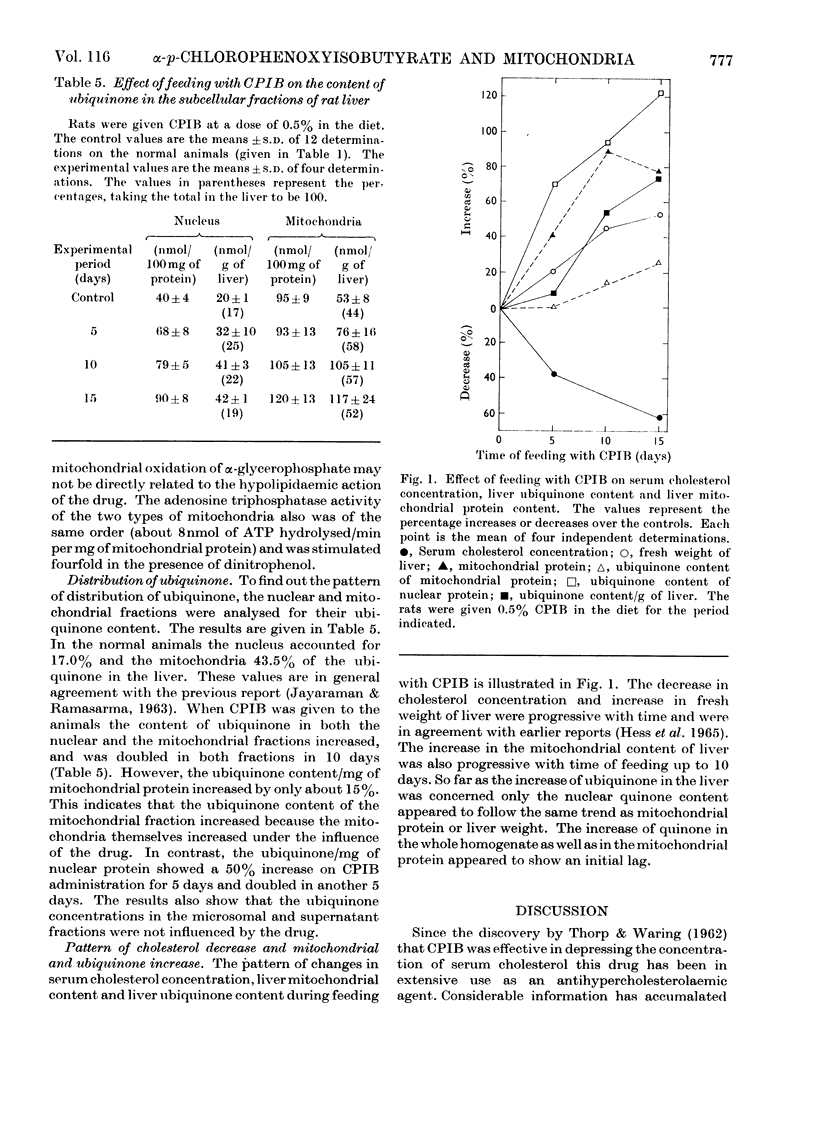
1. The antihypercholesterolaemic drug ethyl α-p-chlorophenoxyisobutyrate when fed to the rat orally or mixed with the diet increased the content of mitochondria in the liver by 50–100%. Other subcellular fractions did not show any significant change. 2. In oxidative activity, respiratory control and phosphorylating ability no significant difference was observed between the mitochondria isolated from the livers of the drug-treated rats and those from normal animals. 3. In agreement with earlier reports, administration of the drug depressed the concentration of serum cholesterol and increased liver weight and the liver content of ubiquinone. However, the increase of ubiquinone was greater in the nuclear than in the mitochondrial protein.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVOY D. R., SWYRYD E. A., GOULD R. G. EFFECTS OF ALPHA-P-CHLOROPHENOXYISOBUTYRYL ETHYL ESTER (CPIB) WITH AND WITHOUT ANDROSTERONE ON CHOLESTEROL BIOSYNTHESIS IN RAT LIVER. J Lipid Res. 1965 Jul;6:369–376. [PubMed] [Google Scholar]
- Brunner G., Bygrave F. L. Microsomal marker enzymes and their limitations in distinguishing the outer membrane of rat liver mitochondria from the microsomes. Eur J Biochem. 1969 Apr;8(4):530–534. doi: 10.1111/j.1432-1033.1969.tb00558.x. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. III. The steady state. J Biol Chem. 1955 Nov;217(1):409–427. [PubMed] [Google Scholar]
- HOGEBOOM G. H., SCHNEIDER W. C. Cytochemical studies of mammalian tissues. III. Isocitric dehydrogenase and triphosphopyridine nucleotide-cytochrome c reductase of mouse liver. J Biol Chem. 1950 Oct;186(2):417–427. [PubMed] [Google Scholar]
- Hess R., Stäubli W., Riess W. Nature of the hepatomegalic effect produced by ethyl-chlorophenoxy-isobutyrate in the rat. Nature. 1965 Nov 27;208(5013):856–858. doi: 10.1038/208856a0. [DOI] [PubMed] [Google Scholar]
- JAYARAMAN J., RAMASARMA T. INTRACELLULAR DISTRIBUTION OF COENZYME Q IN RAT LIVER. Arch Biochem Biophys. 1963 Nov;103:258–266. doi: 10.1016/0003-9861(63)90403-x. [DOI] [PubMed] [Google Scholar]
- Jayaraman J., Cotman C., Mahler H. R., Sharp C. W. Biochemical correlates of respiratory deficiency. VII. Glucose repression. Arch Biochem Biophys. 1966 Sep 26;116(1):224–251. doi: 10.1016/0003-9861(66)90029-4. [DOI] [PubMed] [Google Scholar]
- Krishnaiah K. V., Inamdar A. R., Ramasarma T. Regulation of steroidogenesis by ubiquinone. Biochem Biophys Res Commun. 1967 May 25;27(4):474–478. doi: 10.1016/s0006-291x(67)80009-3. [DOI] [PubMed] [Google Scholar]
- Krishnaiah K. V., Joshi V. C., Ramasarma T. Effect of dietary cholesterol and ubiquinone on isoprene synthesis in rat liver. Arch Biochem Biophys. 1967 Jul;121(1):147–153. doi: 10.1016/0003-9861(67)90019-7. [DOI] [PubMed] [Google Scholar]
- Krishnaiah K. V., Ramasarma T. Effect of alpha-p-chlorophenoxyisobutyrate on the metabolism of isoprenoid compounds in the rat. Biochem J. 1970 Feb;116(3):321–327. doi: 10.1042/bj1160321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE M. J., WHITEHOUSE M. W. THE EFFECT OF BILE SALTS AND SOME BILE-SALT ANALOGUES ON THE OXIDATION OF CHOLESTEROL BY LIVER MITOCHONDRIA. Biochem J. 1963 Nov;89:189–195. doi: 10.1042/bj0890189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osanai M., Rembold H. Entwicklungsabhängige mitochondriale Enzymaktivitäten bei den Kasten der Honigbiene. Biochim Biophys Acta. 1968 Jul 16;162(1):22–31. doi: 10.1016/0005-2728(68)90210-7. [DOI] [PubMed] [Google Scholar]
- Osorio C., Walton K. W., Browne C. H., West D., Whystock P. The effect of chlorphenoxyisobutyrate ('Atromid-S') on the biliary excretion and distribution of thyroxine in the rat. Biochem Pharmacol. 1965 Oct;14(10):1479–1481. doi: 10.1016/0006-2952(65)90181-4. [DOI] [PubMed] [Google Scholar]
- PAGET G. E. EXPERIMENTAL STUDIES OF THE TOXICITY OF ATROMID WITH PARTICULAR REFERENCE TO FINE STRUCTURAL CHANGES IN THE LIVERS OF RODENTS. J Atheroscler Res. 1963 Sep-Dec;3:729–736. doi: 10.1016/s0368-1319(63)80059-9. [DOI] [PubMed] [Google Scholar]
- Phillips W. E., Lakshmanan M. R., Brien R. L. Effect of chlorophenoxyisobutyrate on rat liver non-saponifiables. Can J Physiol Pharmacol. 1968 Jan;46(1):81–84. doi: 10.1139/y68-014. [DOI] [PubMed] [Google Scholar]
- Ruegamer W. R., Ryan N. T., Richert D. A., Westerfeld W. W. The effects of p-chlorophenoxyisobutyrate on the turnover rate and distribution of thyroid hormone in the rat. Biochem Pharmacol. 1969 Mar;18(3):613–624. doi: 10.1016/0006-2952(69)90086-0. [DOI] [PubMed] [Google Scholar]
- SWANSON M. A. Phosphatases of liver. I. Glucose-6-phosphatase. J Biol Chem. 1950 Jun;184(2):647–659. [PubMed] [Google Scholar]
- THORP J. M., WARING W. S. Modification of metabolism and distribution of lipids by ethyl chlorophenoxyisobutyrate. Nature. 1962 Jun 9;194:948–949. doi: 10.1038/194948a0. [DOI] [PubMed] [Google Scholar]
- Westerfeld W. W., Richert D. A., Ruegamer W. R. The role of the thyroid hormone in the effect of p-chlorophenoxyisobutyrate in rats. Biochem Pharmacol. 1968 Jun;17(6):1003–1016. doi: 10.1016/0006-2952(68)90359-6. [DOI] [PubMed] [Google Scholar]


