Abstract
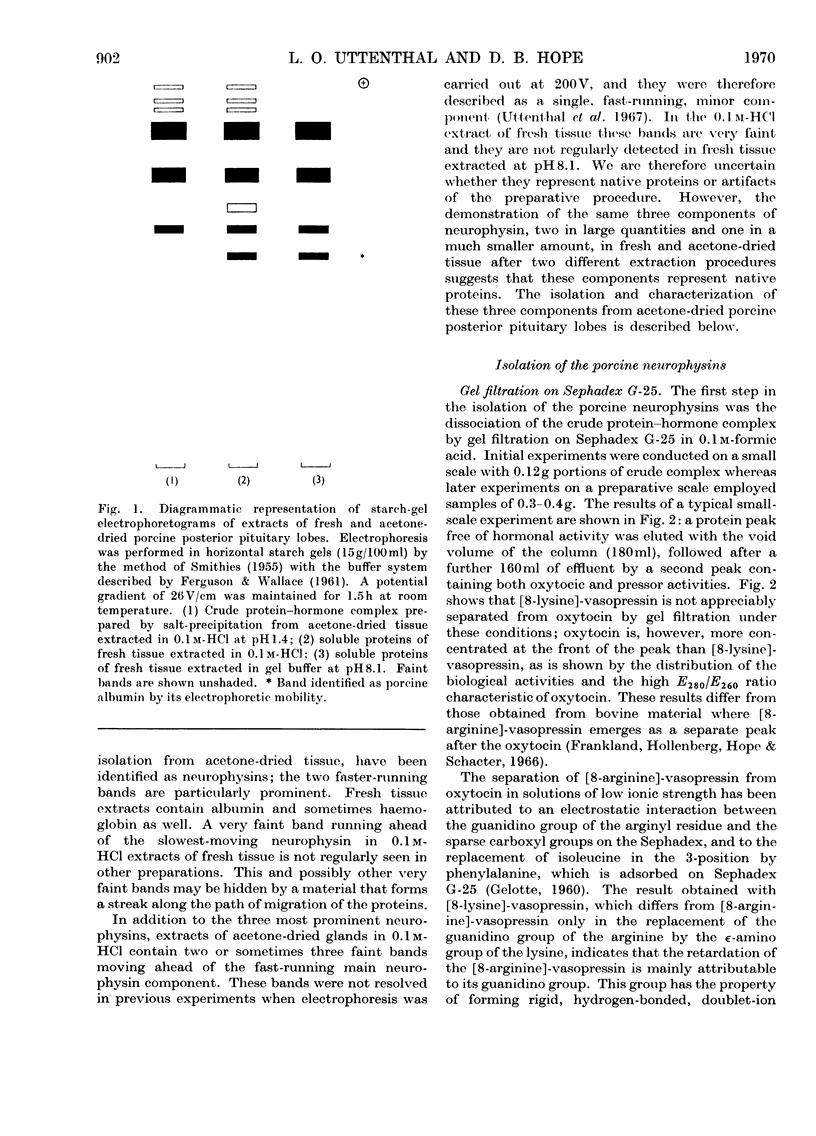
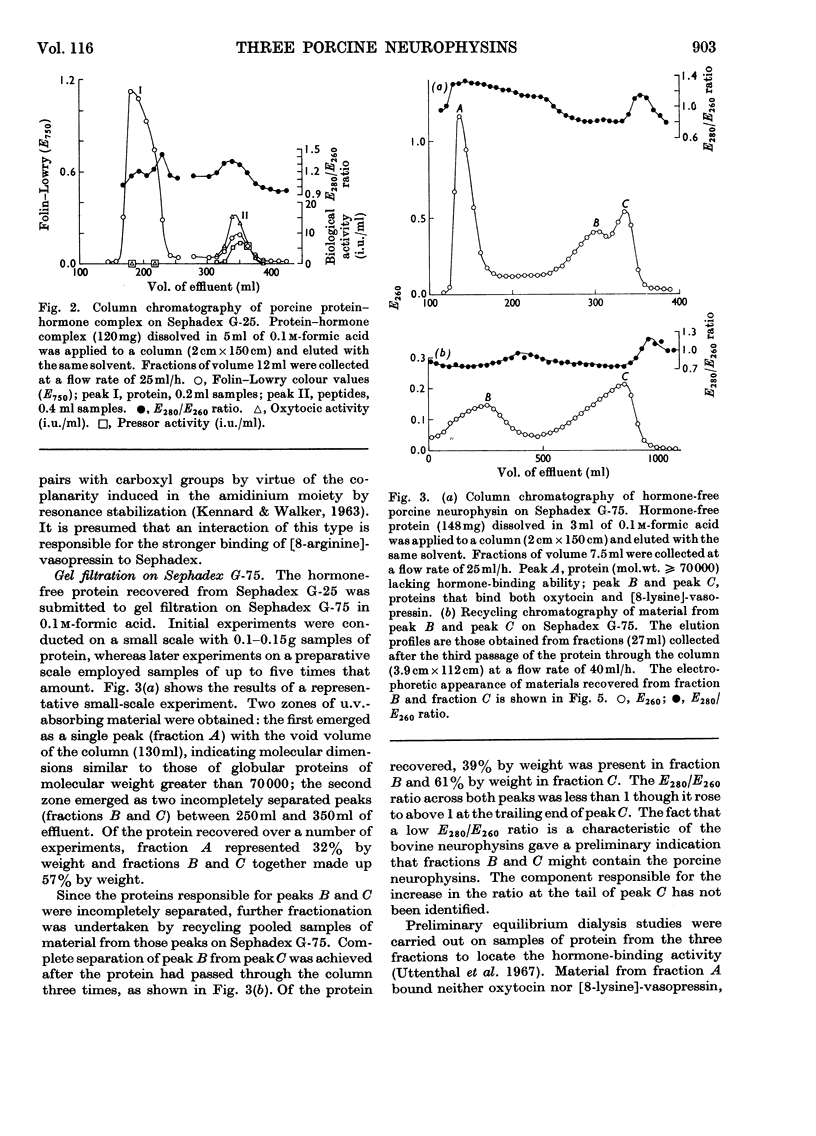
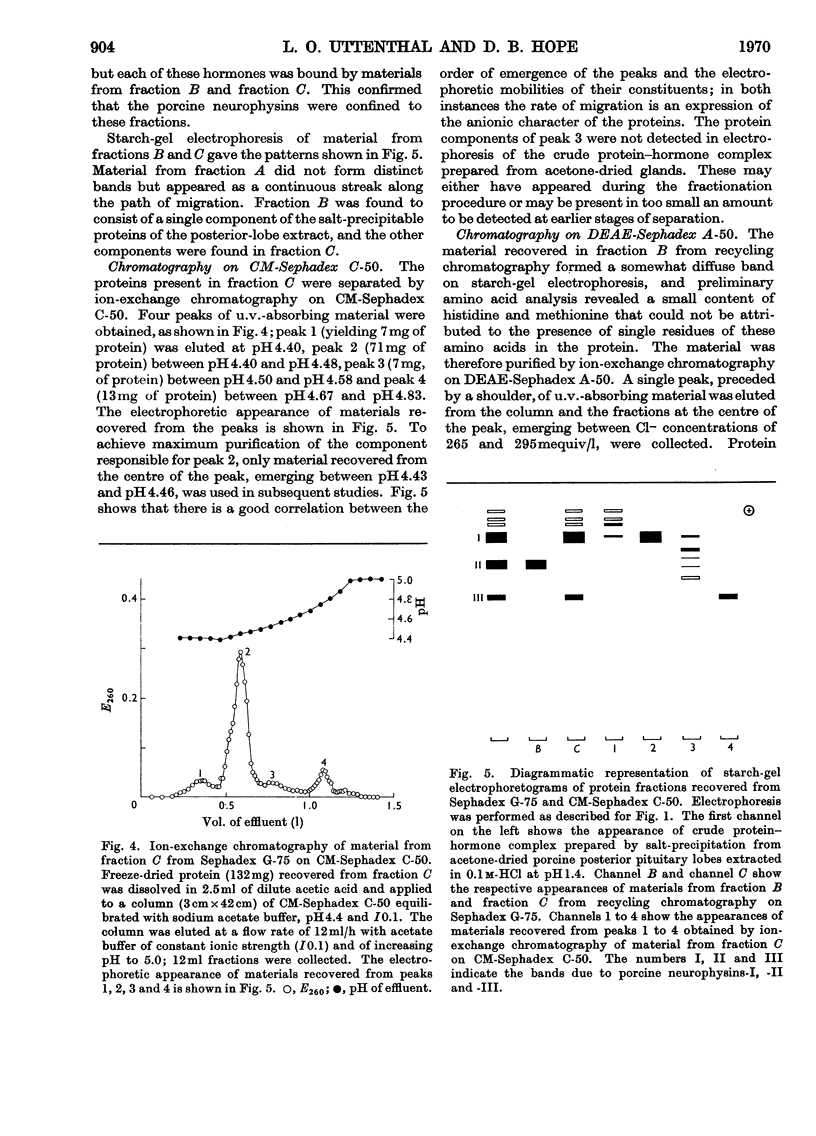
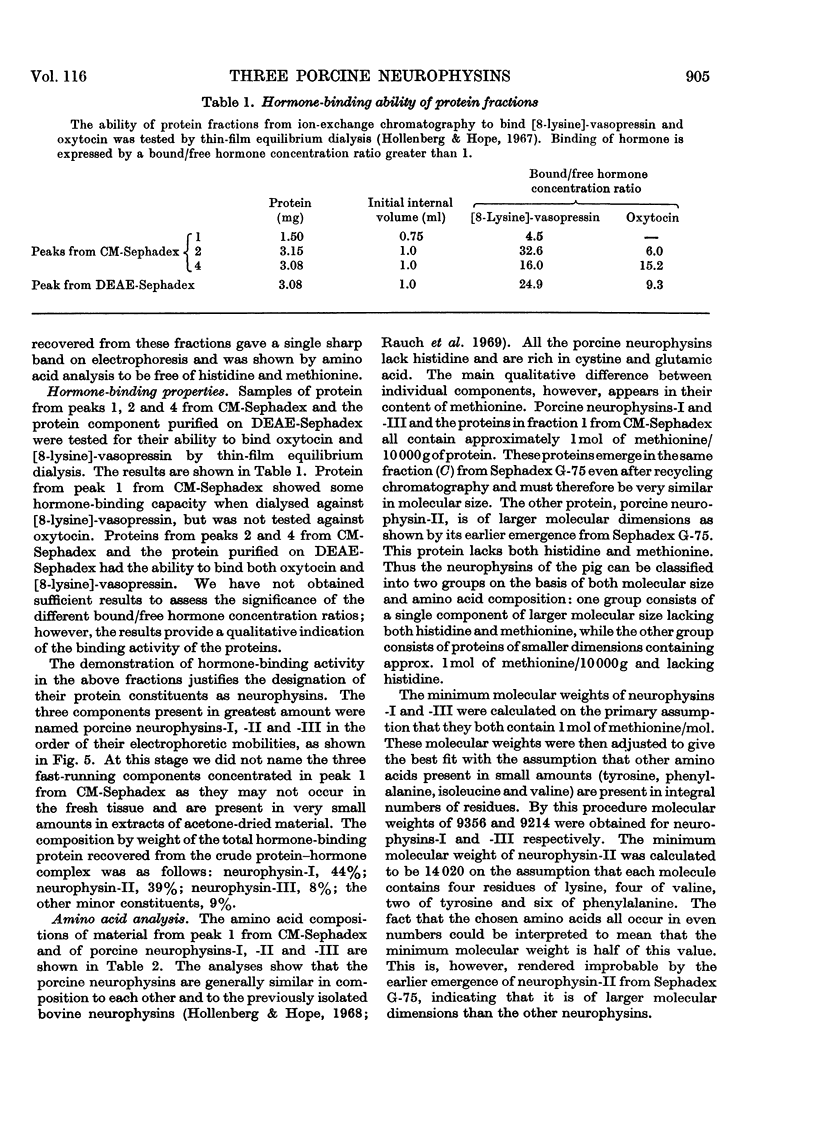
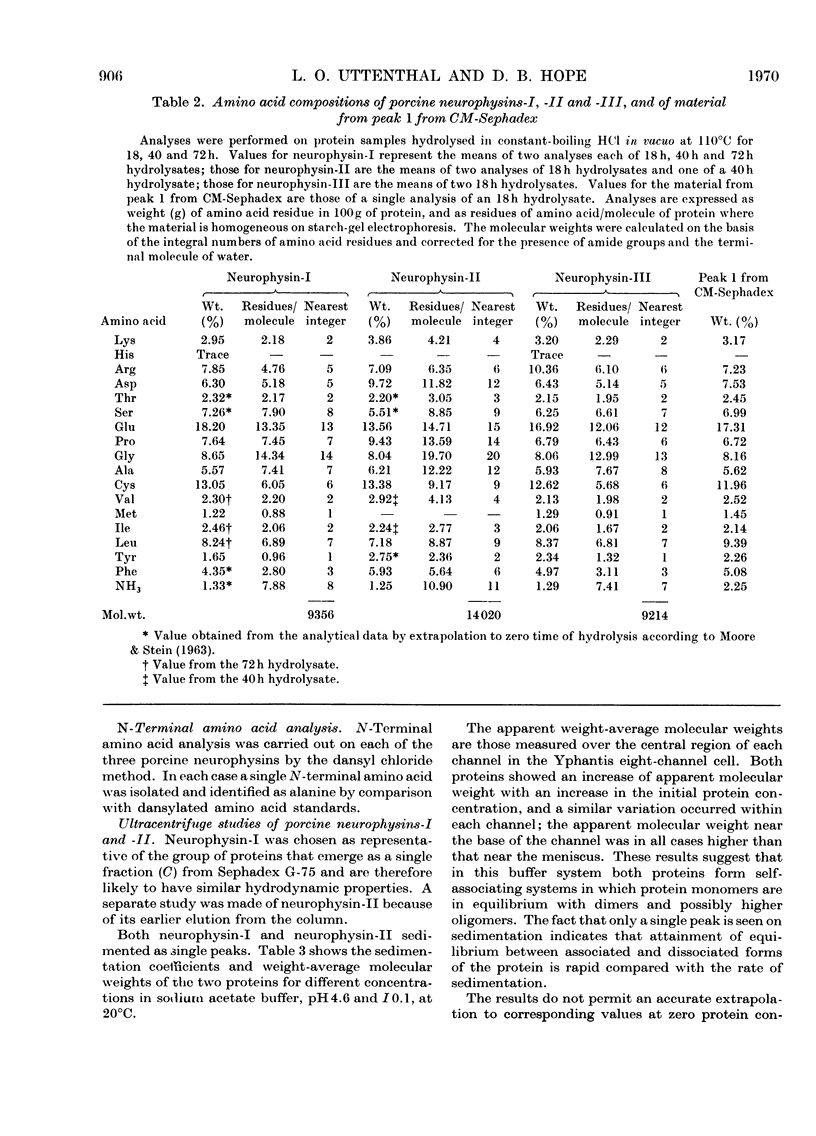
1. Three neurophysins, proteins that bind the polypeptide hormones oxytocin and vasopressin, have been isolated from acetone-dried porcine posterior pituitary lobes. The proteins have been named porcine neurophysins-I, -II and -III in order of their electrophoretic mobilities at pH8.1. 2. Electrophoretic comparison of the purified proteins, which are homogeneous on starch-gel electrophoresis, with the soluble proteins of fresh porcine posterior pituitary lobes extracted in 0.1m-HCl and in buffer pH8.1 suggests that the isolated proteins are native to the fresh tissue. 3. Neurophysins-I and -II are present in similar amounts in the tissue, whereas neurophysin-III is present only in small quantities. Acetone-dried tissue also contains traces of other hormone-binding neurophysin components. 4. All the neurophysins can bind both oxytocin and [8-lysine]-vasopressin. 5. The apparent molecular weights of the neurophysins increase with increasing protein concentration as measured by equilibrium sedimentation in the ultracentrifuge. 6. Neurophysins-I and -III are of similar molecular dimensions, contain one residue of methionine per molecule and lack histidine. The minimum molecular weight of neurophysin-I obtained by amino acid analysis is 9360. Neurophysin-II is of larger molecular dimensions than neurophysins-I and -III and can be separated from these by gel filtration on Sephadex G-75. It contains no histidine or methionine, and its minimum molecular weight has been estimated as 14020 by amino acid analysis. 7. Each of the three neurophysins possesses N-terminal alanine. 8. The possible biological significance of the existence of several neurophysins within one species is discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASTWOOD E. B., BARRETT R. J., FRIESEN H. Two metabolically active peptides from porcine pituitary glands. Proc Natl Acad Sci U S A. 1961 Oct 15;47:1525–1530. doi: 10.1073/pnas.47.10.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BANGHAM D. R., MUSSETT M. V. Third international standard for posterior pituitary; re-named third international standard for oxytocic, vasopressor and antidiuretic substances in 1956. Bull World Health Organ. 1958;19(2):325–340. [PMC free article] [PubMed] [Google Scholar]
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- DEKANSKI J. The quantitative assay of vasopressin. Br J Pharmacol Chemother. 1952 Dec;7(4):567–572. doi: 10.1111/j.1476-5381.1952.tb00723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C. R., Hope D. B. The isolation of neurophysin-I and-II from bovine pituitary neurosecretory granules separated on a large scale from other subcellular organelles. Demonstration of slow equilibration of neurosecretory granules during centrifugation in a sucrose density gradient. Biochem J. 1968 Jan;106(2):565–573. doi: 10.1042/bj1060565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C. R., Hope D. B. The isolation of purified neurosecretory granules from bovine pituitary posterior lobes. Comparison of granule protein constituents with those of neurophysin. Biochem J. 1967 Sep;104(3):1082–1088. doi: 10.1042/bj1041082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERGUSON K. A., WALLACE A. L. Starch-gel electrophoresis of anterior pituitary hormones. Nature. 1961 May 13;190:629–630. doi: 10.1038/190629a0. [DOI] [PubMed] [Google Scholar]
- Ferguson D. R., Heller H. Distribution of neurohypophysial hormones in mammals. J Physiol. 1965 Oct;180(4):846–863. doi: 10.1113/jphysiol.1965.sp007735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland B. T., Hollenberg M. D., Hope D. B., Schacter B. A. Dissociation of oxytocin and vasopressin from their carrier protein by chromatography on sephadex G-25. Br J Pharmacol Chemother. 1966 Feb;26(2):502–510. doi: 10.1111/j.1476-5381.1966.tb01931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen H. G., Astwood E. B. Changes in neurohypophysial proteins induced by dehydration and ingestion of saline. Endocrinology. 1967 Feb;80(2):278–287. doi: 10.1210/endo-80-2-278. [DOI] [PubMed] [Google Scholar]
- Ginsburg M., Thomas P. J. The molecular dimensions of porcine neurophysin and some thermodynamic parameters of the reaction with lysine vasopressin. J Physiol. 1969 Mar;201(1):181–199. doi: 10.1113/jphysiol.1969.sp008750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg M. D., Hope D. B. Fractionation of neurophysin by molecular-sieve and ion-exchange chromatography. Biochem J. 1967 Jul;104(1):122–127. doi: 10.1042/bj1040122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg M. D., Hope D. B. The isolation of the native hormone-binding proteins from bovine pituitary posterior lobes. Crystallization of neurophysin-I and-II as complexes with [8-arginine]-vasopressin. Biochem J. 1968 Jan;106(2):557–564. doi: 10.1042/bj1060557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan N. O. Nature of multiple molecular forms of enzymes. Ann N Y Acad Sci. 1968 Jun 14;151(1):382–399. doi: 10.1111/j.1749-6632.1968.tb11902.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MUNSICK R. A. Effect of magnesium ion on the response of the rat uterus to neurohypophysial hormones and analogues. Endocrinology. 1960 Mar;6:451–457. doi: 10.1210/endo-66-3-451. [DOI] [PubMed] [Google Scholar]
- Morse D., Horecker B. L. Thin-layer chromatographic separation of DNS-amino acids. Anal Biochem. 1966 Mar;14(3):429–433. doi: 10.1016/0003-2697(66)90285-5. [DOI] [PubMed] [Google Scholar]
- Rauch R., Hollenberg M. D., Hope D. B. Isolation of a third bovine neurophysin. Biochem J. 1969 Nov;115(3):473–479. doi: 10.1042/bj1150473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch R., Hollenberg M. D., Hope D. B. Isolation of a third neurophysin from bovine pituitary posterior lobes. Biochem J. 1968 Dec;110(3):38P–38P. doi: 10.1042/bj1100038pa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SACHS H., TAKABATAKE Y. EVIDENCE FOR A PRECURSOR IN VASOPRESSIN BIOSYNTHESIS. Endocrinology. 1964 Dec;75:943–948. doi: 10.1210/endo-75-6-943. [DOI] [PubMed] [Google Scholar]
- SMITHIES O. Zone electrophoresis in starch gels: group variations in the serum proteins of normal human adults. Biochem J. 1955 Dec;61(4):629–641. doi: 10.1042/bj0610629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuu T. C., Saffran M. Isolation and characterization of a hormone-binding polypeptide from pig posterior pituitary powder. J Biol Chem. 1969 Jan 25;244(2):482–490. [PubMed] [Google Scholar]
- YPHANSTIS D. A. Rapid determination of molecular weights of peptides and preteins. Ann N Y Acad Sci. 1960 Aug 31;88:586–601. doi: 10.1111/j.1749-6632.1960.tb20055.x. [DOI] [PubMed] [Google Scholar]


