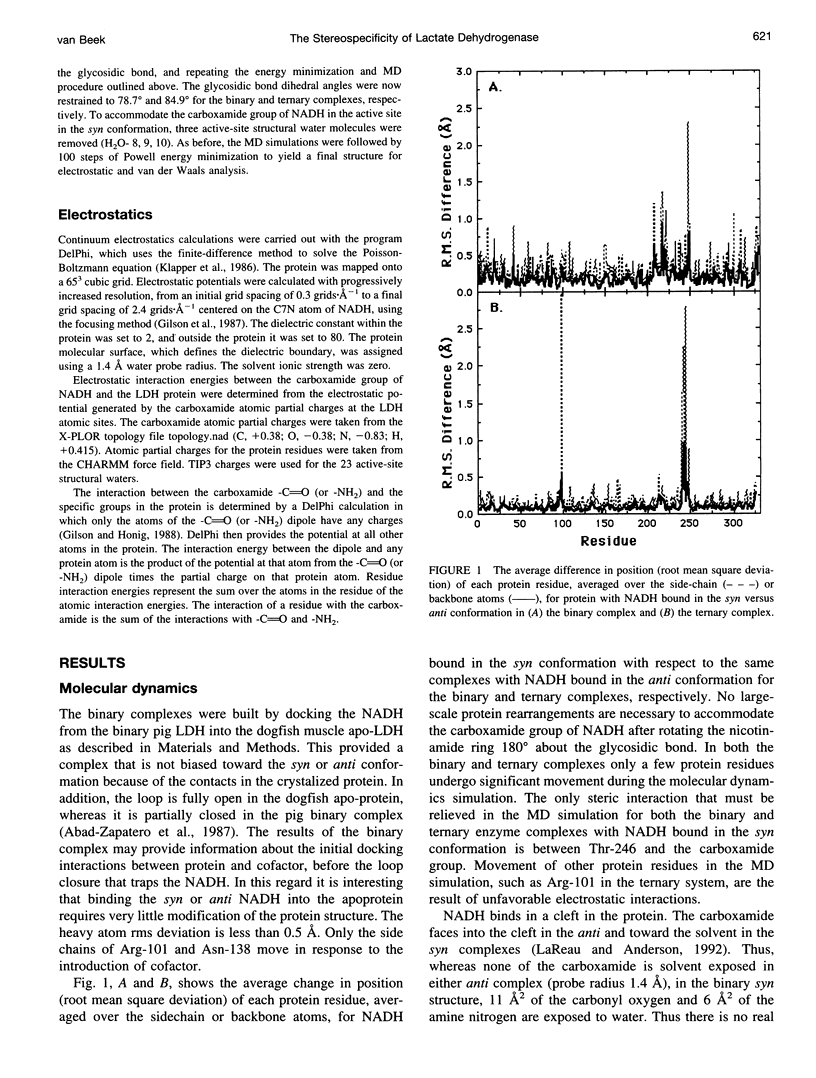
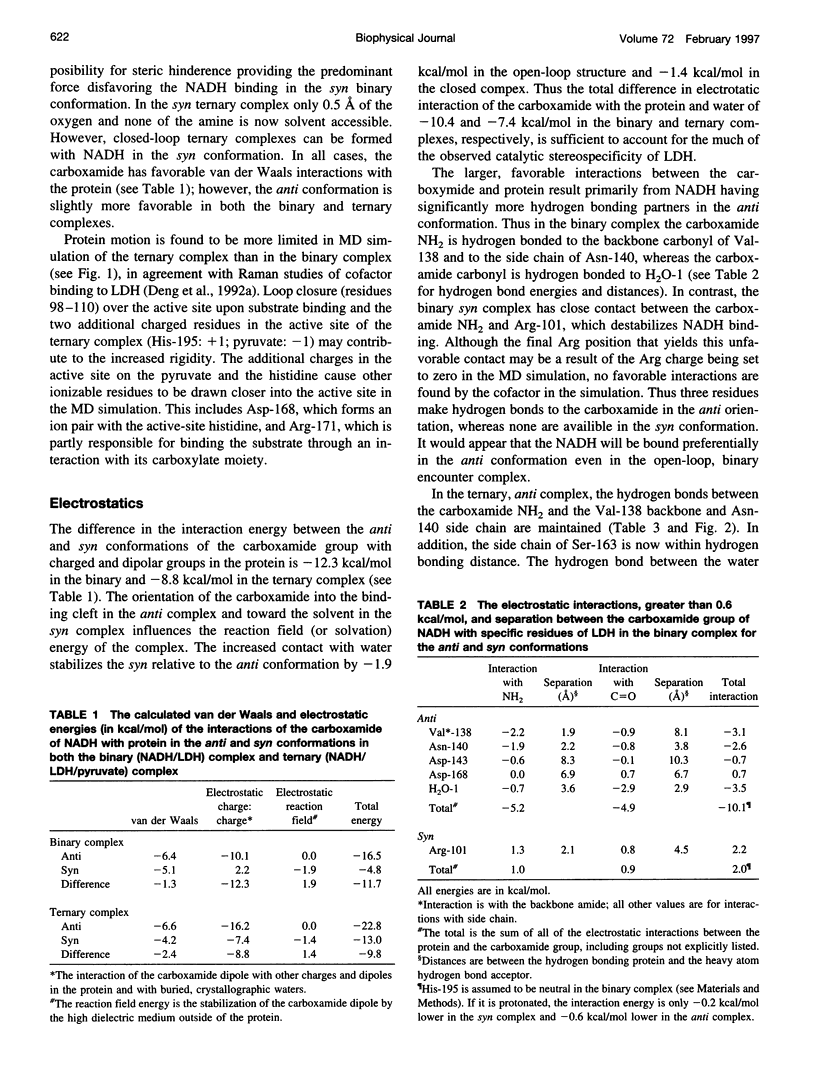
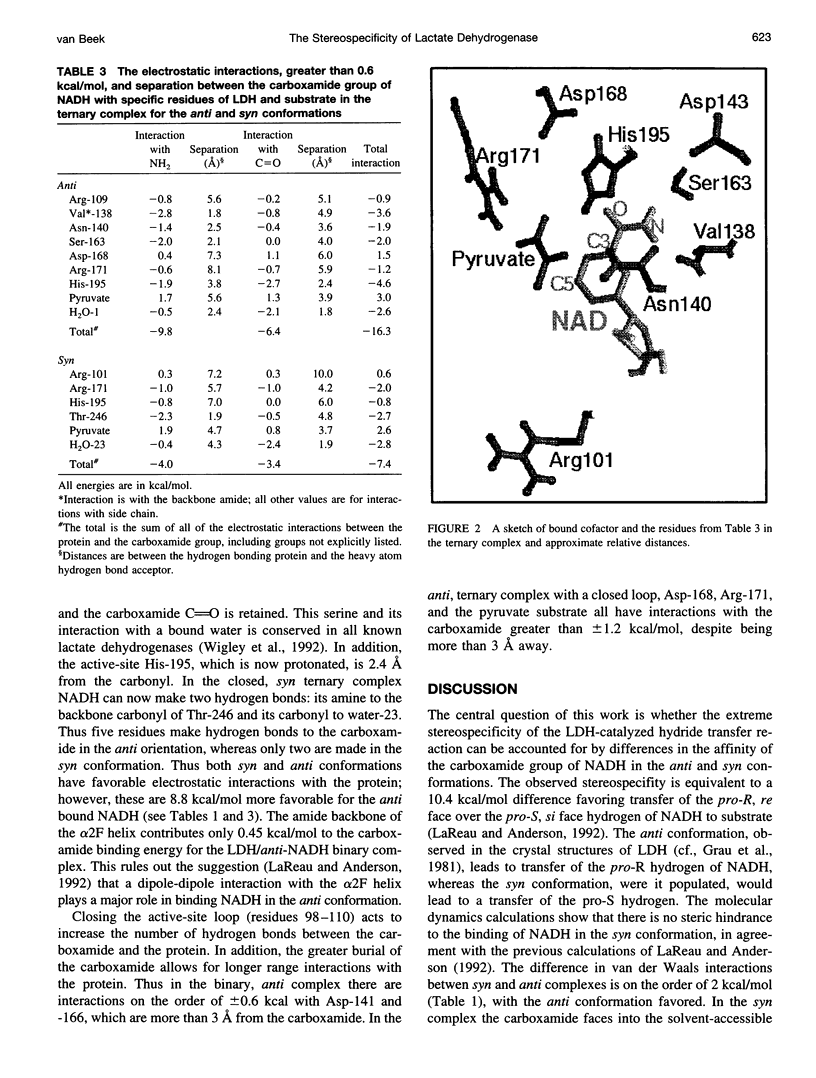
Abstract
Continuum electrostatic calculations in conjunction with molecular dynamics simulations have been used to investigate the source of the stereospecificity in the hydride transfer reaction catalyzed by lactate dehydrogenase (LDH). These studies show that favorable electrostatic interactions between the carboxamide group of the reduced nicotinamide adenine dinucleotide coenzyme and protein residues of the active site of LDH can account for much if not all of the stereospecificity of the LDH-catalyzed reaction, with A-side hydride transfer more than 107 times greater than B-side transfer. Unfavorable steric interactions within the binding complex for B-side transfer are not found.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abad-Zapatero C., Griffith J. P., Sussman J. L., Rossmann M. G. Refined crystal structure of dogfish M4 apo-lactate dehydrogenase. J Mol Biol. 1987 Dec 5;198(3):445–467. doi: 10.1016/0022-2836(87)90293-2. [DOI] [PubMed] [Google Scholar]
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Chandrasekhar K., McPherson A., Jr, Adams M. J., Rossmann M. G. Conformation of coenzyme fragments when bound to lactate dehydrogenase. J Mol Biol. 1973 Jun 5;76(4):503–518. doi: 10.1016/0022-2836(73)90488-9. [DOI] [PubMed] [Google Scholar]
- Clarke A. R., Wigley D. B., Chia W. N., Barstow D., Atkinson T., Holbrook J. J. Site-directed mutagenesis reveals role of mobile arginine residue in lactate dehydrogenase catalysis. Nature. 1986 Dec 18;324(6098):699–702. doi: 10.1038/324699a0. [DOI] [PubMed] [Google Scholar]
- Clarke A. R., Wilks H. M., Barstow D. A., Atkinson T., Chia W. N., Holbrook J. J. An investigation of the contribution made by the carboxylate group of an active site histidine-aspartate couple to binding and catalysis in lactate dehydrogenase. Biochemistry. 1988 Mar 8;27(5):1617–1622. doi: 10.1021/bi00405a034. [DOI] [PubMed] [Google Scholar]
- Deng H., Burgner J., Callender R. Raman spectroscopic studies of NAD coenzymes bound to malate dehydrogenases by difference techniques. Biochemistry. 1991 Sep 10;30(36):8804–8811. doi: 10.1021/bi00100a011. [DOI] [PubMed] [Google Scholar]
- Deng H., Zheng J., Clarke A., Holbrook J. J., Callender R., Burgner J. W., 2nd Source of catalysis in the lactate dehydrogenase system. Ground-state interactions in the enzyme-substrate complex. Biochemistry. 1994 Mar 1;33(8):2297–2305. doi: 10.1021/bi00174a042. [DOI] [PubMed] [Google Scholar]
- Deng H., Zheng J., Sloan D., Burgner J., Callender R. A vibrational analysis of the catalytically important C4-H bonds of NADH bound to lactate or malate dehydrogenase: ground-state effects. Biochemistry. 1992 Jun 2;31(21):5085–5092. doi: 10.1021/bi00136a022. [DOI] [PubMed] [Google Scholar]
- Deng H., Zheng J., Sloan D., Burgner J., Callender R. Classical Raman spectroscopic studies of NADH and NAD+ bound to lactate dehydrogenase by difference techniques. Biochemistry. 1989 Feb 21;28(4):1525–1533. doi: 10.1021/bi00430a016. [DOI] [PubMed] [Google Scholar]
- Dunn C. R., Wilks H. M., Halsall D. J., Atkinson T., Clarke A. R., Muirhead H., Holbrook J. J. Design and synthesis of new enzymes based on the lactate dehydrogenase framework. Philos Trans R Soc Lond B Biol Sci. 1991 May 29;332(1263):177–184. doi: 10.1098/rstb.1991.0047. [DOI] [PubMed] [Google Scholar]
- Fisher H. F., Adija D. L., Cross D. G. Dehydrogenae-reduced coenzyme difference spectra, their resolution and relationship to the stereospecificity of hydrogen transfer. Biochemistry. 1969 Nov;8(11):4424–4431. doi: 10.1021/bi00839a030. [DOI] [PubMed] [Google Scholar]
- Gilson M. K., Honig B. Calculation of the total electrostatic energy of a macromolecular system: solvation energies, binding energies, and conformational analysis. Proteins. 1988;4(1):7–18. doi: 10.1002/prot.340040104. [DOI] [PubMed] [Google Scholar]
- Grau U. M., Trommer W. E., Rossmann M. G. Structure of the active ternary complex of pig heart lactate dehydrogenase with S-lac-NAD at 2.7 A resolution. J Mol Biol. 1981 Sep 15;151(2):289–307. doi: 10.1016/0022-2836(81)90516-7. [DOI] [PubMed] [Google Scholar]
- Klapper I., Hagstrom R., Fine R., Sharp K., Honig B. Focusing of electric fields in the active site of Cu-Zn superoxide dismutase: effects of ionic strength and amino-acid modification. Proteins. 1986 Sep;1(1):47–59. doi: 10.1002/prot.340010109. [DOI] [PubMed] [Google Scholar]
- LaReau R. D., Anderson V. E. An inquiry into the source of stereospecificity of lactate dehydrogenase using substrate analogues and molecular modeling. Biochemistry. 1992 May 5;31(17):4174–4180. doi: 10.1021/bi00132a004. [DOI] [PubMed] [Google Scholar]
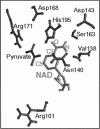
- LaReau R. D., Anderson V. E. Lactate dehydrogenase displays absolute stereospecificity in the transfer of the prochiral hydrogen of NADH. J Biol Chem. 1989 Sep 15;264(26):15338–15343. [PubMed] [Google Scholar]
- Wigley D. B., Gamblin S. J., Turkenburg J. P., Dodson E. J., Piontek K., Muirhead H., Holbrook J. J. Structure of a ternary complex of an allosteric lactate dehydrogenase from Bacillus stearothermophilus at 2.5 A resolution. J Mol Biol. 1992 Jan 5;223(1):317–335. doi: 10.1016/0022-2836(92)90733-z. [DOI] [PubMed] [Google Scholar]
- Zheng J., Chen Y. Q., Callender R. A study of the binding of NADP coenzymes to dihydrofolate reductase by raman difference spectroscopy. Eur J Biochem. 1993 Jul 1;215(1):9–16. doi: 10.1111/j.1432-1033.1993.tb18001.x. [DOI] [PubMed] [Google Scholar]