Abstract
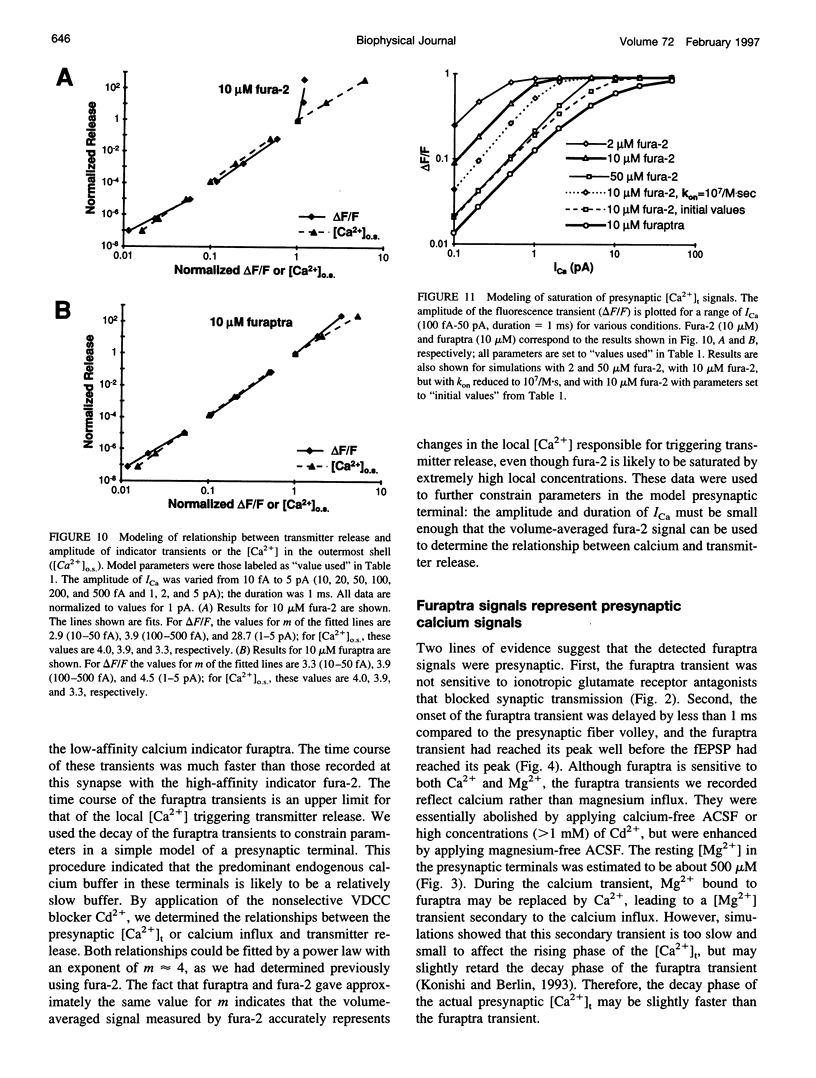
The relationship between presynaptic calcium transients ([Ca2+]t) and transmitter release evoked by a single stimulus was both investigated experimentally and modeled at a mammalian central synapse, the CA3 to CA1 pyramidal cell synapse in guinea pig hippocampal slices. In the present study, we compared the low-affinity calcium indicator furaptra with the higher-affinity indicator fura-2. The 10-90% rise time of the furaptra transient was 2.4 ms compared to 7.8 ms with fura-2; the half-decay time (τ1/2) was 30 ms for furaptra, compared to 238 ms for fura-2. The half-width of the calcium influx was 1.8 ms with furaptra, which provides an upper limit to the duration of the calcium current (ICa) evoked by an action potential. Modeling the decay time course of the furaptra transients led to the conclusion that the predominant endogenous calcium buffer in these terminals must have relatively slow kinetics (kon < 105/M·s), although the presence of small amounts of fast buffers cannot be excluded. The relationship between the [Ca2+]t measured with furaptra and the postsynaptic response was the same as previously observed with fura-2: the postsynaptic response was proportional to about the fourth power (m ≈ 4) of the amplitude of either [Ca2+]t or calcium influx. Thus, although fura-2 may be locally saturated by the high local [Ca2+] responsible for transmitter release, the volume-averaged fura-2 signal accurately reflects changes in this local concentration. The result that both indicators gave similar values for the power m constrains the amplitude of calcium influx in our model: ICa < 1 pA for 1 ms.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler E. M., Augustine G. J., Duffy S. N., Charlton M. P. Alien intracellular calcium chelators attenuate neurotransmitter release at the squid giant synapse. J Neurosci. 1991 Jun;11(6):1496–1507. doi: 10.1523/JNEUROSCI.11-06-01496.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine G. J., Adler E. M., Charlton M. P. The calcium signal for transmitter secretion from presynaptic nerve terminals. Ann N Y Acad Sci. 1991;635:365–381. doi: 10.1111/j.1749-6632.1991.tb36505.x. [DOI] [PubMed] [Google Scholar]
- Augustine G. J., Charlton M. P. Calcium dependence of presynaptic calcium current and post-synaptic response at the squid giant synapse. J Physiol. 1986 Dec;381:619–640. doi: 10.1113/jphysiol.1986.sp016347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine G. J., Charlton M. P., Smith S. J. Calcium entry and transmitter release at voltage-clamped nerve terminals of squid. J Physiol. 1985 Oct;367:163–181. doi: 10.1113/jphysiol.1985.sp015819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Hollingworth S. Fura-2 calcium transients in frog skeletal muscle fibres. J Physiol. 1988 Sep;403:151–192. doi: 10.1113/jphysiol.1988.sp017244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin J. R., Bassani J. W., Bers D. M. Intrinsic cytosolic calcium buffering properties of single rat cardiac myocytes. Biophys J. 1994 Oct;67(4):1775–1787. doi: 10.1016/S0006-3495(94)80652-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin J. R., Konishi M. Ca2+ transients in cardiac myocytes measured with high and low affinity Ca2+ indicators. Biophys J. 1993 Oct;65(4):1632–1647. doi: 10.1016/S0006-3495(93)81211-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst J. G., Helmchen F., Sakmann B. Pre- and postsynaptic whole-cell recordings in the medial nucleus of the trapezoid body of the rat. J Physiol. 1995 Dec 15;489(Pt 3):825–840. doi: 10.1113/jphysiol.1995.sp021095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst J. G., Sakmann B. Calcium influx and transmitter release in a fast CNS synapse. Nature. 1996 Oct 3;383(6599):431–434. doi: 10.1038/383431a0. [DOI] [PubMed] [Google Scholar]
- Charlton M. P., Smith S. J., Zucker R. S. Role of presynaptic calcium ions and channels in synaptic facilitation and depression at the squid giant synapse. J Physiol. 1982 Feb;323:173–193. doi: 10.1113/jphysiol.1982.sp014067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. D., Lester R. A., Tong G., Jahr C. E., Westbrook G. L. The time course of glutamate in the synaptic cleft. Science. 1992 Nov 27;258(5087):1498–1501. doi: 10.1126/science.1359647. [DOI] [PubMed] [Google Scholar]
- Cohen M. W., Jones O. T., Angelides K. J. Distribution of Ca2+ channels on frog motor nerve terminals revealed by fluorescent omega-conotoxin. J Neurosci. 1991 Apr;11(4):1032–1039. doi: 10.1523/JNEUROSCI.11-04-01032.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittman J. S., Regehr W. G. Contributions of calcium-dependent and calcium-independent mechanisms to presynaptic inhibition at a cerebellar synapse. J Neurosci. 1996 Mar 1;16(5):1623–1633. doi: 10.1523/JNEUROSCI.16-05-01623.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Heidelberger R., Heinemann C., Neher E., Matthews G. Calcium dependence of the rate of exocytosis in a synaptic terminal. Nature. 1994 Oct 6;371(6497):513–515. doi: 10.1038/371513a0. [DOI] [PubMed] [Google Scholar]
- Kao J. P., Tsien R. Y. Ca2+ binding kinetics of fura-2 and azo-1 from temperature-jump relaxation measurements. Biophys J. 1988 Apr;53(4):635–639. doi: 10.1016/S0006-3495(88)83142-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Further study of the role of calcium in synaptic transmission. J Physiol. 1970 May;207(3):789–801. doi: 10.1113/jphysiol.1970.sp009095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M., Berlin J. R. Ca transients in cardiac myocytes measured with a low affinity fluorescent indicator, furaptra. Biophys J. 1993 Apr;64(4):1331–1343. doi: 10.1016/S0006-3495(93)81494-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagnado L., Cervetto L., McNaughton P. A. Calcium homeostasis in the outer segments of retinal rods from the tiger salamander. J Physiol. 1992 Sep;455:111–142. doi: 10.1113/jphysiol.1992.sp019293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landò L., Zucker R. S. Ca2+ cooperativity in neurosecretion measured using photolabile Ca2+ chelators. J Neurophysiol. 1994 Aug;72(2):825–830. doi: 10.1152/jn.1994.72.2.825. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Silver R. B. Microdomains of high calcium concentration in a presynaptic terminal. Science. 1992 May 1;256(5057):677–679. doi: 10.1126/science.1350109. [DOI] [PubMed] [Google Scholar]
- Mackenzie P. J., Umemiya M., Murphy T. H. Ca2+ imaging of CNS axons in culture indicates reliable coupling between single action potentials and distal functional release sites. Neuron. 1996 Apr;16(4):783–795. doi: 10.1016/s0896-6273(00)80098-7. [DOI] [PubMed] [Google Scholar]
- Miller A., Hu B. A molecular model of low-voltage-activated calcium conductance. J Neurophysiol. 1995 Jun;73(6):2349–2356. doi: 10.1152/jn.1995.73.6.2349. [DOI] [PubMed] [Google Scholar]
- Mintz I. M., Sabatini B. L., Regehr W. G. Calcium control of transmitter release at a cerebellar synapse. Neuron. 1995 Sep;15(3):675–688. doi: 10.1016/0896-6273(95)90155-8. [DOI] [PubMed] [Google Scholar]
- Neher E., Augustine G. J. Calcium gradients and buffers in bovine chromaffin cells. J Physiol. 1992 May;450:273–301. doi: 10.1113/jphysiol.1992.sp019127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. The use of fura-2 for estimating Ca buffers and Ca fluxes. Neuropharmacology. 1995 Nov;34(11):1423–1442. doi: 10.1016/0028-3908(95)00144-u. [DOI] [PubMed] [Google Scholar]
- Raju B., Murphy E., Levy L. A., Hall R. D., London R. E. A fluorescent indicator for measuring cytosolic free magnesium. Am J Physiol. 1989 Mar;256(3 Pt 1):C540–C548. doi: 10.1152/ajpcell.1989.256.3.C540. [DOI] [PubMed] [Google Scholar]
- Regehr W. G., Atluri P. P. Calcium transients in cerebellar granule cell presynaptic terminals. Biophys J. 1995 May;68(5):2156–2170. doi: 10.1016/S0006-3495(95)80398-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regehr W. G., Delaney K. R., Tank D. W. The role of presynaptic calcium in short-term enhancement at the hippocampal mossy fiber synapse. J Neurosci. 1994 Feb;14(2):523–537. doi: 10.1523/JNEUROSCI.14-02-00523.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regehr W. G., Tank D. W. Selective fura-2 loading of presynaptic terminals and nerve cell processes by local perfusion in mammalian brain slice. J Neurosci Methods. 1991 Apr;37(2):111–119. doi: 10.1016/0165-0270(91)90121-f. [DOI] [PubMed] [Google Scholar]
- Roberts W. M., Jacobs R. A., Hudspeth A. J. Colocalization of ion channels involved in frequency selectivity and synaptic transmission at presynaptic active zones of hair cells. J Neurosci. 1990 Nov;10(11):3664–3684. doi: 10.1523/JNEUROSCI.10-11-03664.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W. M. Spatial calcium buffering in saccular hair cells. Nature. 1993 May 6;363(6424):74–76. doi: 10.1038/363074a0. [DOI] [PubMed] [Google Scholar]
- Robitaille R., Adler E. M., Charlton M. P. Strategic location of calcium channels at transmitter release sites of frog neuromuscular synapses. Neuron. 1990 Dec;5(6):773–779. doi: 10.1016/0896-6273(90)90336-e. [DOI] [PubMed] [Google Scholar]
- Sala F., Hernández-Cruz A. Calcium diffusion modeling in a spherical neuron. Relevance of buffering properties. Biophys J. 1990 Feb;57(2):313–324. doi: 10.1016/S0006-3495(90)82533-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. J., Buchanan J., Osses L. R., Charlton M. P., Augustine G. J. The spatial distribution of calcium signals in squid presynaptic terminals. J Physiol. 1993 Dec;472:573–593. doi: 10.1113/jphysiol.1993.sp019963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E. F. Single calcium channels and acetylcholine release at a presynaptic nerve terminal. Neuron. 1993 Dec;11(6):1007–1011. doi: 10.1016/0896-6273(93)90214-c. [DOI] [PubMed] [Google Scholar]
- Thomas P., Surprenant A., Almers W. Cytosolic Ca2+, exocytosis, and endocytosis in single melanotrophs of the rat pituitary. Neuron. 1990 Nov;5(5):723–733. doi: 10.1016/0896-6273(90)90226-6. [DOI] [PubMed] [Google Scholar]
- WESTRUM L. E., BLACKSTAD T. W. An electron microscopic study of the stratum radiatum of the rat hippocampus (regio superior, CA 1) with particular emphasis on synaptology. J Comp Neurol. 1962 Dec;119:281–309. doi: 10.1002/cne.901190303. [DOI] [PubMed] [Google Scholar]
- Wheeler D. B., Randall A., Tsien R. W. Changes in action potential duration alter reliance of excitatory synaptic transmission on multiple types of Ca2+ channels in rat hippocampus. J Neurosci. 1996 Apr 1;16(7):2226–2237. doi: 10.1523/JNEUROSCI.16-07-02226.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L. G., Saggau P. Adenosine inhibits evoked synaptic transmission primarily by reducing presynaptic calcium influx in area CA1 of hippocampus. Neuron. 1994 May;12(5):1139–1148. doi: 10.1016/0896-6273(94)90321-2. [DOI] [PubMed] [Google Scholar]
- Wu L. G., Saggau P. Block of multiple presynaptic calcium channel types by omega-conotoxin-MVIIC at hippocampal CA3 to CA1 synapses. J Neurophysiol. 1995 May;73(5):1965–1972. doi: 10.1152/jn.1995.73.5.1965. [DOI] [PubMed] [Google Scholar]
- Wu L. G., Saggau P. GABAB receptor-mediated presynaptic inhibition in guinea-pig hippocampus is caused by reduction of presynaptic Ca2+ influx. J Physiol. 1995 Jun 15;485(Pt 3):649–657. doi: 10.1113/jphysiol.1995.sp020759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L. G., Saggau P. Pharmacological identification of two types of presynaptic voltage-dependent calcium channels at CA3-CA1 synapses of the hippocampus. J Neurosci. 1994 Sep;14(9):5613–5622. doi: 10.1523/JNEUROSCI.14-09-05613.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L. G., Saggau P. Presynaptic calcium is increased during normal synaptic transmission and paired-pulse facilitation, but not in long-term potentiation in area CA1 of hippocampus. J Neurosci. 1994 Feb;14(2):645–654. doi: 10.1523/JNEUROSCI.14-02-00645.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawo H., Chuhma N. Omega-conotoxin-sensitive and -resistant transmitter release from the chick ciliary presynaptic terminal. J Physiol. 1994 Jun 15;477(Pt 3):437–448. doi: 10.1113/jphysiol.1994.sp020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Neher E. Mobile and immobile calcium buffers in bovine adrenal chromaffin cells. J Physiol. 1993 Sep;469:245–273. doi: 10.1113/jphysiol.1993.sp019813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker R. S., Delaney K. R., Mulkey R., Tank D. W. Presynaptic calcium in transmitter release and posttetanic potentiation. Ann N Y Acad Sci. 1991;635:191–207. doi: 10.1111/j.1749-6632.1991.tb36492.x. [DOI] [PubMed] [Google Scholar]
- Zucker R. S., Fogelson A. L. Relationship between transmitter release and presynaptic calcium influx when calcium enters through discrete channels. Proc Natl Acad Sci U S A. 1986 May;83(9):3032–3036. doi: 10.1073/pnas.83.9.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Baldawi N. F., Abercrombie R. F. Cytoplasmic calcium buffer capacity determined with Nitr-5 and DM-nitrophen. Cell Calcium. 1995 Jun;17(6):409–421. doi: 10.1016/0143-4160(95)90087-x. [DOI] [PubMed] [Google Scholar]



