Abstract
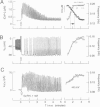
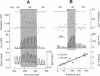
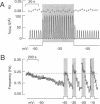
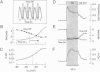
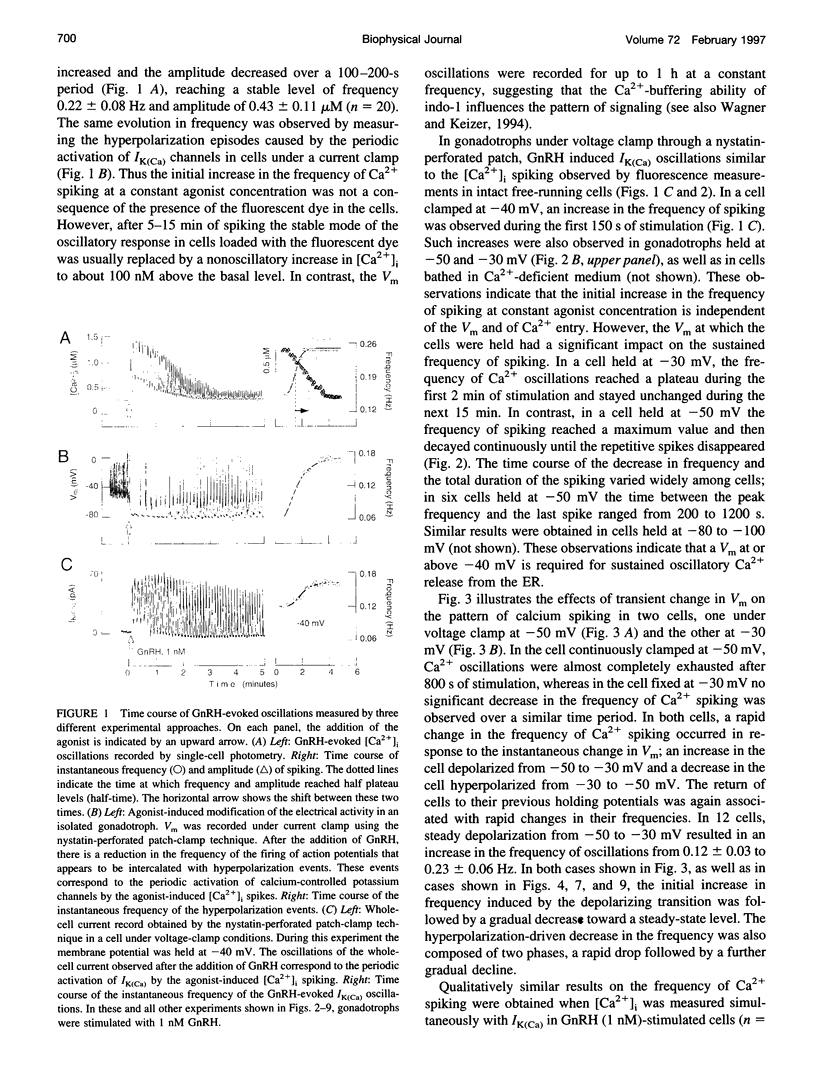
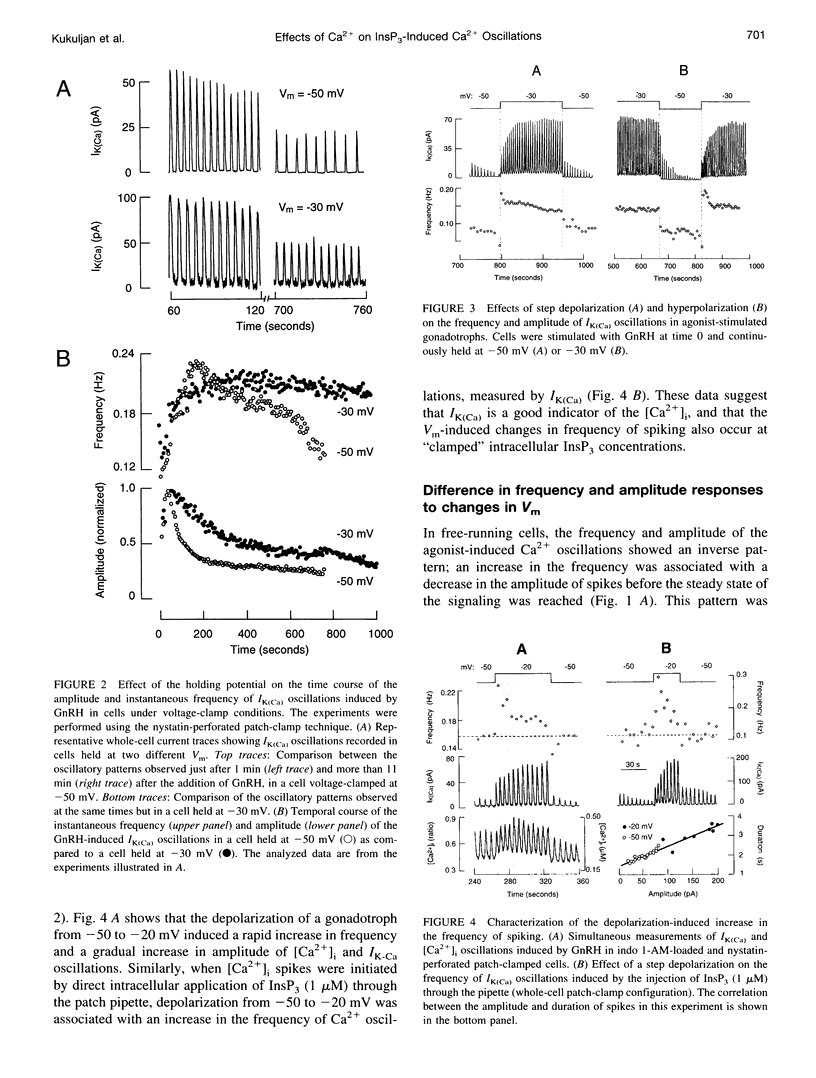
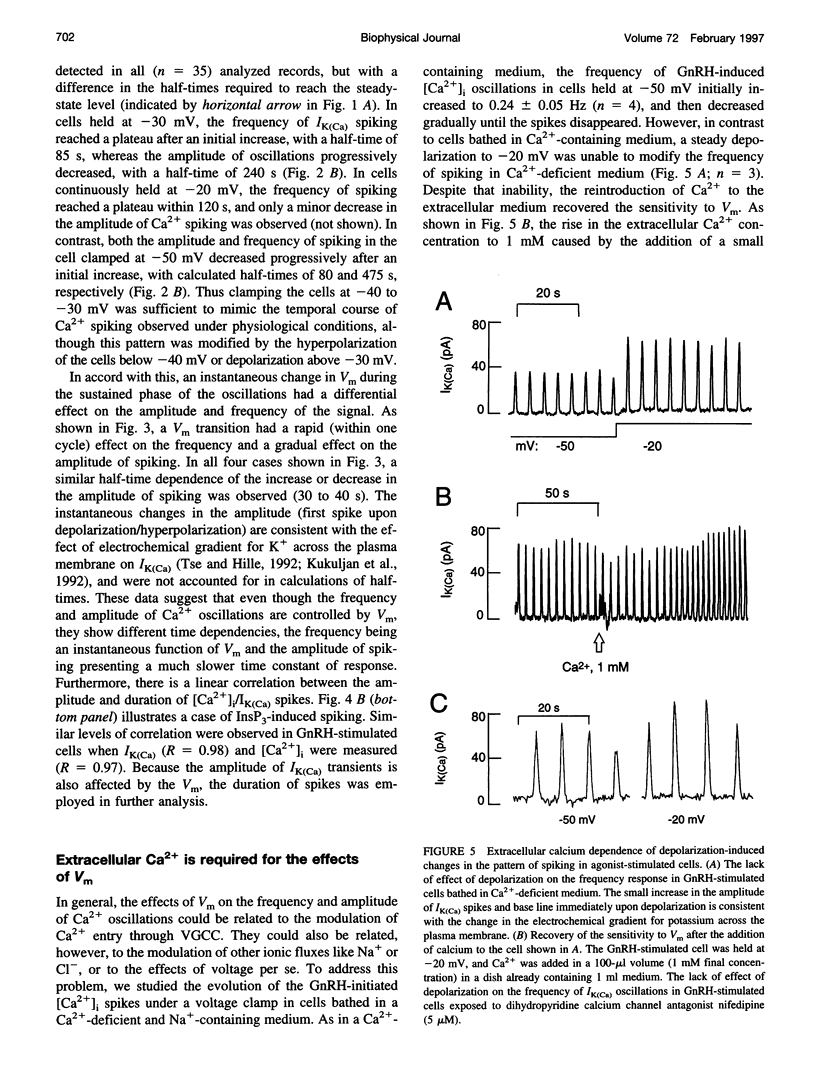
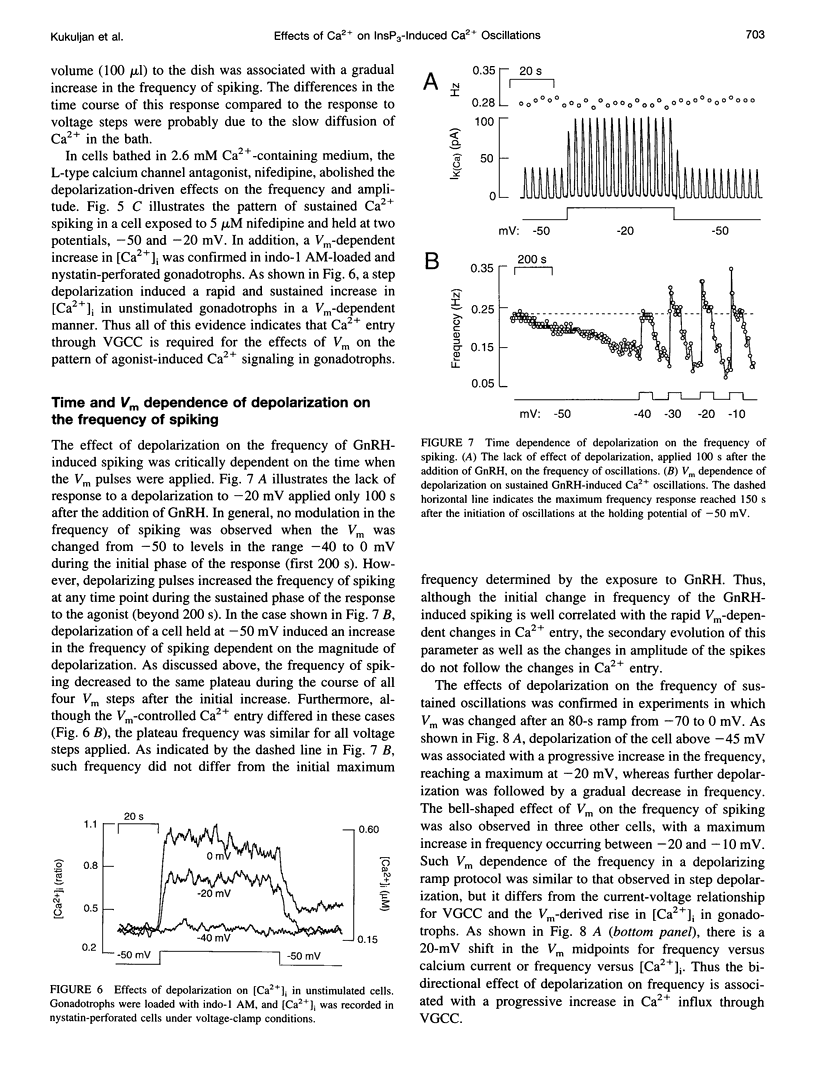
Inositol 1,4,5-trisphosphate (InsP3) binds to its receptor channels and causes liberation of Ca2+ from intracellular stores, frequently in an oscillatory manner. In addition to InsP3, the activation and inactivation properties of these intracellular channels are controlled by Ca2+. We studied the influence of Ca2+ entry on the kinetics of InsP3-triggered oscillations in cytosolic calcium ([Ca2+]i) in gonadotrophs stimulated with gonadotropin-releasing hormone, an agonist that activates InsP3 production. The natural expression of voltage-gated Ca2+ channels (VGCC) in these cells was employed to manipulate Ca2+ entry by voltage clamping the cells at different membrane potentials (Vm). Under physiological conditions, the frequency of the GnRH-induced oscillations increased with time, while the amplitude decreased, until both reached stable values. However, in cells with Vm held at -50 mV or lower, both parameters progressively decreased until the signal was abolished. These effects were reverted by a depolarization of the membrane positive to -45 mV in both agonist- and InsP3-stimulated gonadotrophs. Depolarization also led to an increase in the fraction of time during which the [Ca2+]i remained elevated; this effect originated from both an increase in the mean duration of spikes and a decrease in the interval between spikes. The frequency and amplitude of spiking depended on the activity of VGCC, but displayed different temporal courses and voltage relationships. The depolarization-driven recovery of the frequency was instantaneous, whereas the recovery of the amplitude of spiking was more gradual. The midpoints of the Vm sensitivity curve for amplitude and duration of spiking (-15 mV) were close to the value observed for L-type Ca2+ current and for depolarization-induced increase in [Ca2+]i, whereas this parameter was much lower (-35 mV) for interval between spikes and frequency of oscillations. These observations are compatible with at least two distinct effects of Ca2+ entry on the sustained [Ca2+]i oscillations. Calcium influx facilitates its liberation from intracellular stores by a direct and instantaneous action on the release mechanism. It also magnifies the Ca2+ signal and decreases the frequency because of its gradual effect on the reloading of intracellular stores.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bezprozvanny I., Watras J., Ehrlich B. E. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991 Jun 27;351(6329):751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- De Young G. W., Keizer J. A single-pool inositol 1,4,5-trisphosphate-receptor-based model for agonist-stimulated oscillations in Ca2+ concentration. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9895–9899. doi: 10.1073/pnas.89.20.9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisle S., Krause K. H., Denning G., Potter B. V., Welsh M. J. Effect of inositol trisphosphate and calcium on oscillating elevations of intracellular calcium in Xenopus oocytes. J Biol Chem. 1990 Jul 15;265(20):11726–11730. [PubMed] [Google Scholar]
- Emons G., Nill J., Sturm R., Ortmann O. Effects of progesterone on gonadotropin-releasing hormone receptor concentration in cultured estrogen-primed female rat pituitary cells. J Steroid Biochem Mol Biol. 1992 Sep;42(8):831–839. doi: 10.1016/0960-0760(92)90091-v. [DOI] [PubMed] [Google Scholar]
- Finch E. A., Turner T. J., Goldin S. M. Calcium as a coagonist of inositol 1,4,5-trisphosphate-induced calcium release. Science. 1991 Apr 19;252(5004):443–446. doi: 10.1126/science.2017683. [DOI] [PubMed] [Google Scholar]
- Girard S., Clapham D. Acceleration of intracellular calcium waves in Xenopus oocytes by calcium influx. Science. 1993 Apr 9;260(5105):229–232. doi: 10.1126/science.8385801. [DOI] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M., Endo M. Calcium-dependent immediate feedback control of inositol 1,4,5-triphosphate-induced Ca2+ release. Nature. 1992 Nov 5;360(6399):76–78. doi: 10.1038/360076a0. [DOI] [PubMed] [Google Scholar]
- Keizer J., Li Y. X., Stojilković S., Rinzel J. InsP3-induced Ca2+ excitability of the endoplasmic reticulum. Mol Biol Cell. 1995 Aug;6(8):945–951. doi: 10.1091/mbc.6.8.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukuljan M., Rojas E., Catt K. J., Stojilkovic S. S. Membrane potential regulates inositol 1,4,5-trisphosphate-controlled cytoplasmic Ca2+ oscillations in pituitary gonadotrophs. J Biol Chem. 1994 Feb 18;269(7):4860–4865. [PubMed] [Google Scholar]
- Kukuljan M., Stojilković S. S., Rojas E., Catt K. J. Apamin-sensitive potassium channels mediate agonist-induced oscillations of membrane potential in pituitary gonadotrophs. FEBS Lett. 1992 Apr 13;301(1):19–22. doi: 10.1016/0014-5793(92)80201-q. [DOI] [PubMed] [Google Scholar]
- Lechleiter J. D., Clapham D. E. Molecular mechanisms of intracellular calcium excitability in X. laevis oocytes. Cell. 1992 Apr 17;69(2):283–294. doi: 10.1016/0092-8674(92)90409-6. [DOI] [PubMed] [Google Scholar]
- Leong D. A., Thorner M. O. A potential code of luteinizing hormone-releasing hormone-induced calcium ion responses in the regulation of luteinizing hormone secretion among individual gonadotropes. J Biol Chem. 1991 May 15;266(14):9016–9022. [PubMed] [Google Scholar]
- Naor Z., Azrad A., Limor R., Zakut H., Lotan M. Gonadotropin-releasing hormone activates a rapid Ca2+-independent phosphodiester hydrolysis of polyphosphoinositides in pituitary gonadotrophs. J Biol Chem. 1986 Sep 25;261(27):12506–12512. [PubMed] [Google Scholar]
- Parker I., Ivorra I. Inhibition by Ca2+ of inositol trisphosphate-mediated Ca2+ liberation: a possible mechanism for oscillatory release of Ca2+. Proc Natl Acad Sci U S A. 1990 Jan;87(1):260–264. doi: 10.1073/pnas.87.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I., Yao Y., Ilyin V. Fast kinetics of calcium liberation induced in Xenopus oocytes by photoreleased inositol trisphosphate. Biophys J. 1996 Jan;70(1):222–237. doi: 10.1016/S0006-3495(96)79565-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne R., Walz B., Levy S., Fein A. The localization of calcium release by inositol trisphosphate in Limulus photoreceptors and its control by negative feedback. Philos Trans R Soc Lond B Biol Sci. 1988 Jul 26;320(1199):359–379. doi: 10.1098/rstb.1988.0082. [DOI] [PubMed] [Google Scholar]
- Rawlings S. R., Berry D. J., Leong D. A. Evidence for localized calcium mobilization and influx in single rat gonadotropes. J Biol Chem. 1991 Nov 25;266(33):22755–22760. [PubMed] [Google Scholar]
- Rooney T. A., Sass E. J., Thomas A. P. Agonist-induced cytosolic calcium oscillations originate from a specific locus in single hepatocytes. J Biol Chem. 1990 Jun 25;265(18):10792–10796. [PubMed] [Google Scholar]
- Sealfon S. C., Laws S. C., Wu J. C., Gillo B., Miller W. L. Hormonal regulation of gonadotropin-releasing hormone receptors and messenger RNA activity in ovine pituitary culture. Mol Endocrinol. 1990 Dec;4(12):1980–1987. doi: 10.1210/mend-4-12-1980. [DOI] [PubMed] [Google Scholar]
- Shangold G. A., Murphy S. N., Miller R. J. Gonadotropin-releasing hormone-induced Ca2+ transients in single identified gonadotropes require both intracellular Ca2+ mobilization and Ca2+ influx. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6566–6570. doi: 10.1073/pnas.85.17.6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojilkovic S. S., Tomic M., Kukuljan M., Catt K. J. Control of calcium spiking frequency in pituitary gonadotrophs by a single-pool cytoplasmic oscillator. Mol Pharmacol. 1994 May;45(5):1013–1021. [PubMed] [Google Scholar]
- Stojilković S. S., Kukuljan M., Iida T., Rojas E., Catt K. J. Integration of cytoplasmic calcium and membrane potential oscillations maintains calcium signaling in pituitary gonadotrophs. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4081–4085. doi: 10.1073/pnas.89.9.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojilković S. S., Kukuljan M., Tomić M., Rojas E., Catt K. J. Mechanism of agonist-induced [Ca2+]i oscillations in pituitary gonadotrophs. J Biol Chem. 1993 Apr 15;268(11):7713–7720. [PubMed] [Google Scholar]
- Tse A., Hille B. GnRH-induced Ca2+ oscillations and rhythmic hyperpolarizations of pituitary gonadotropes. Science. 1992 Jan 24;255(5043):462–464. doi: 10.1126/science.1734523. [DOI] [PubMed] [Google Scholar]
- Tse A., Tse F. W., Hille B. Modulation of Ca2+ oscillation and apamin-sensitive, Ca2+-activated K+ current in rat gonadotropes. Pflugers Arch. 1995 Sep;430(5):645–652. doi: 10.1007/BF00386158. [DOI] [PubMed] [Google Scholar]
- Vergara L. A., Stojilkovic S. S., Rojas E. GnRH-induced cytosolic calcium oscillations in pituitary gonadotrophs: phase resetting by membrane depolarization. Biophys J. 1995 Oct;69(4):1606–1614. doi: 10.1016/S0006-3495(95)80033-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J., Keizer J. Effects of rapid buffers on Ca2+ diffusion and Ca2+ oscillations. Biophys J. 1994 Jul;67(1):447–456. doi: 10.1016/S0006-3495(94)80500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakui M., Potter B. V., Petersen O. H. Pulsatile intracellular calcium release does not depend on fluctuations in inositol trisphosphate concentration. Nature. 1989 May 25;339(6222):317–320. doi: 10.1038/339317a0. [DOI] [PubMed] [Google Scholar]
- Zhang B. X., Muallem S. Feedback inhibition of Ca2+ release by Ca2+ is the underlying mechanism of agonist-evoked intracellular Ca2+ oscillations in pancreatic acinar cells. J Biol Chem. 1992 Dec 5;267(34):24387–24393. [PubMed] [Google Scholar]
- Zhao H., Loessberg P. A., Sachs G., Muallem S. Regulation of intracellular Ca2+ oscillation in AR42J cells. J Biol Chem. 1990 Dec 5;265(34):20856–20862. [PubMed] [Google Scholar]