Abstract
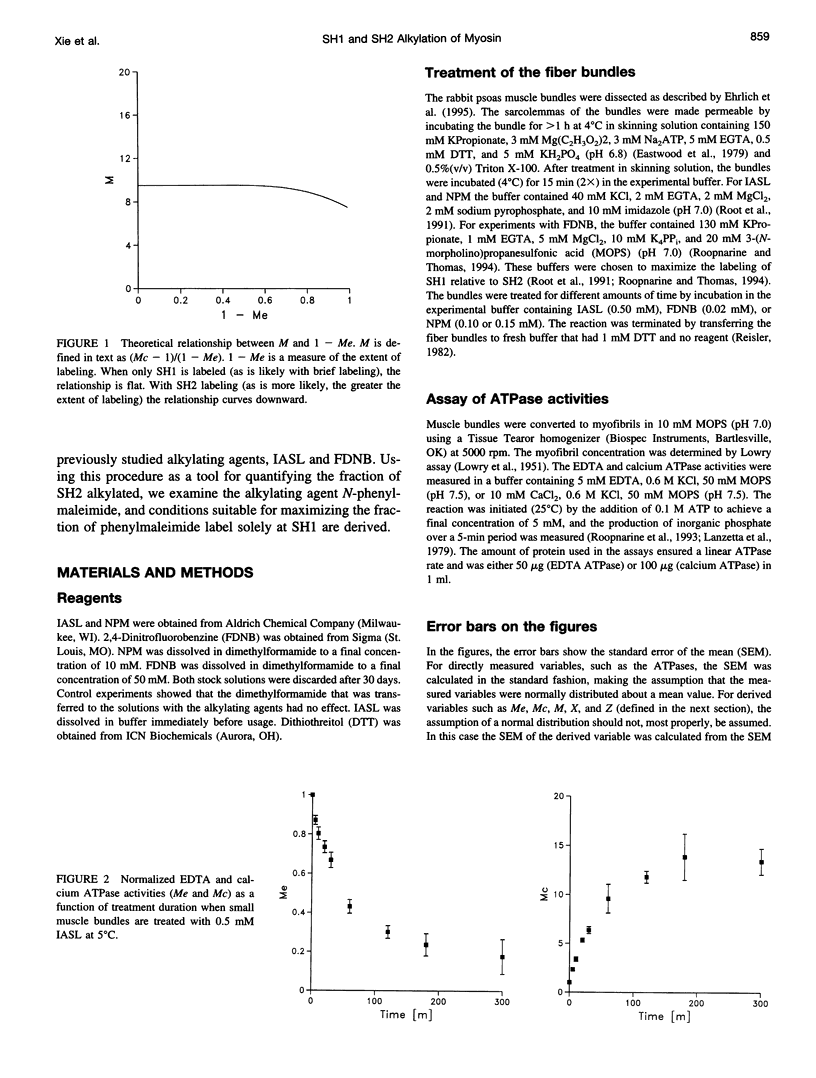
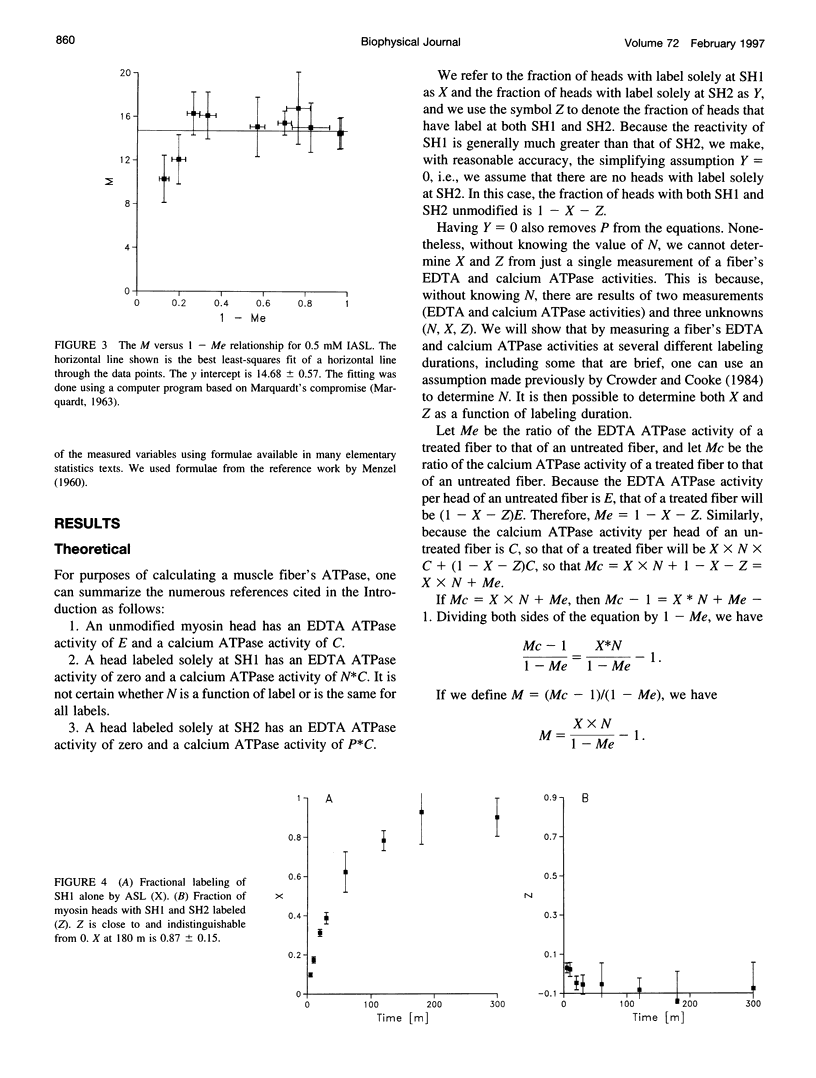
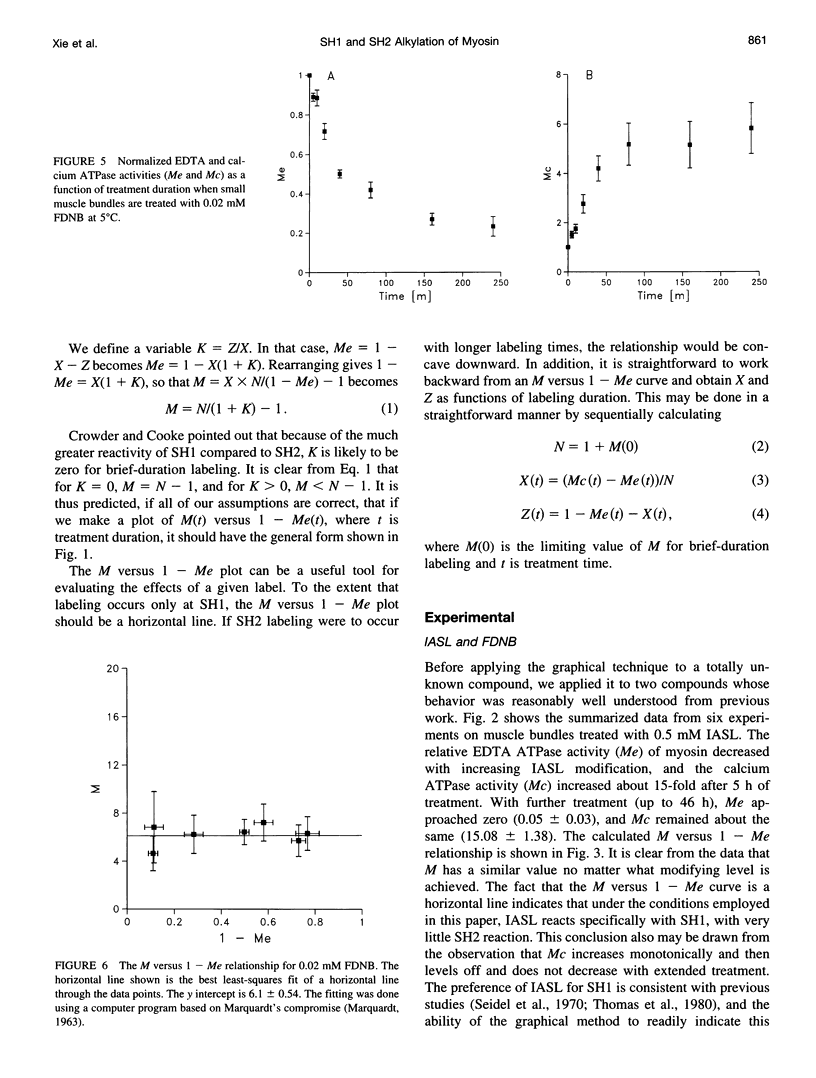
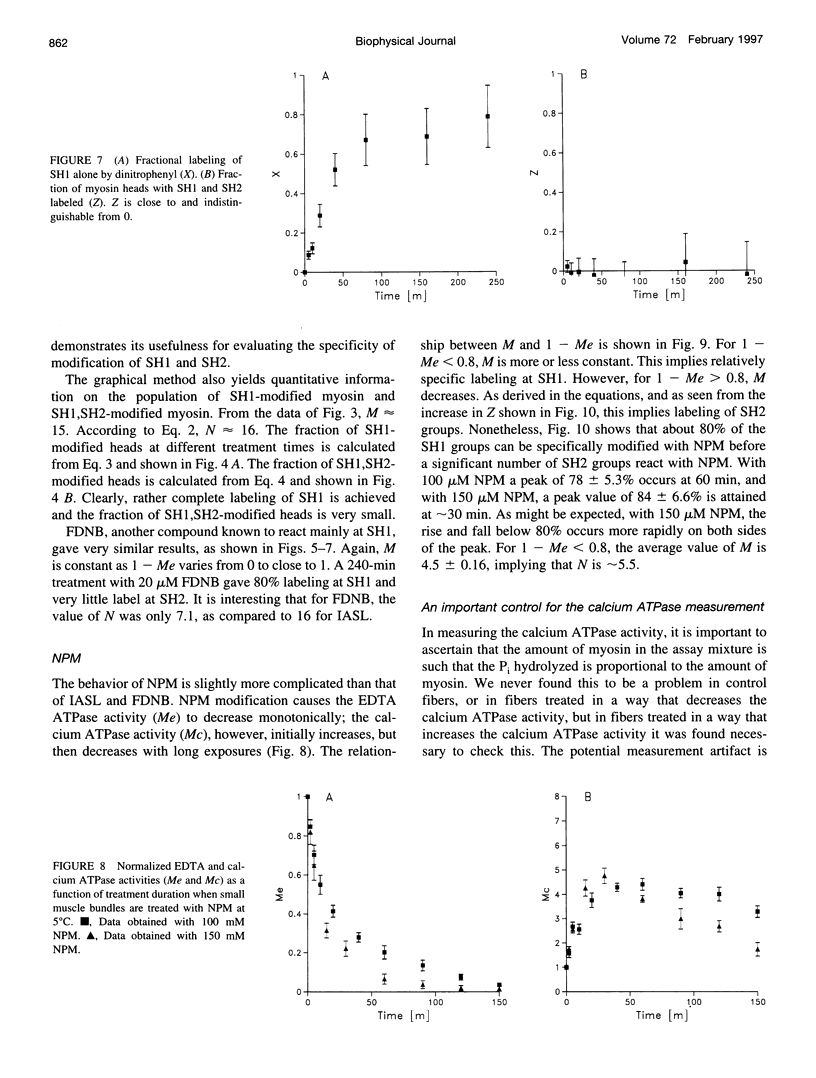
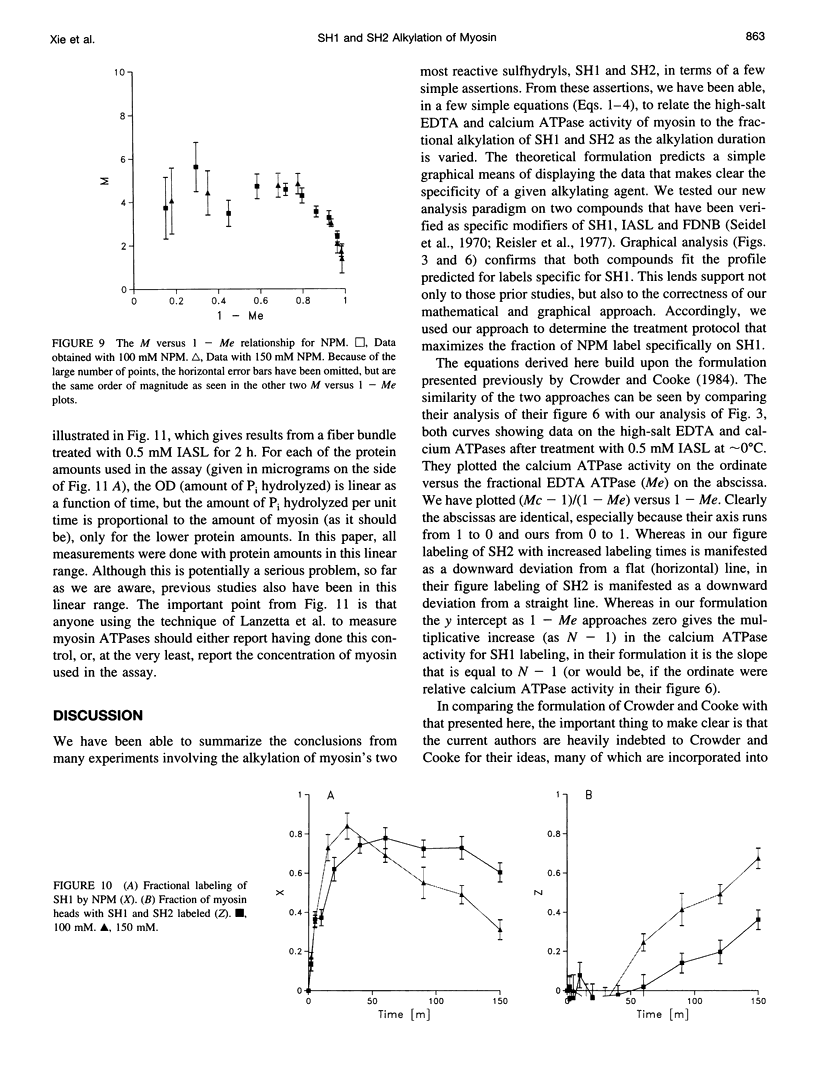
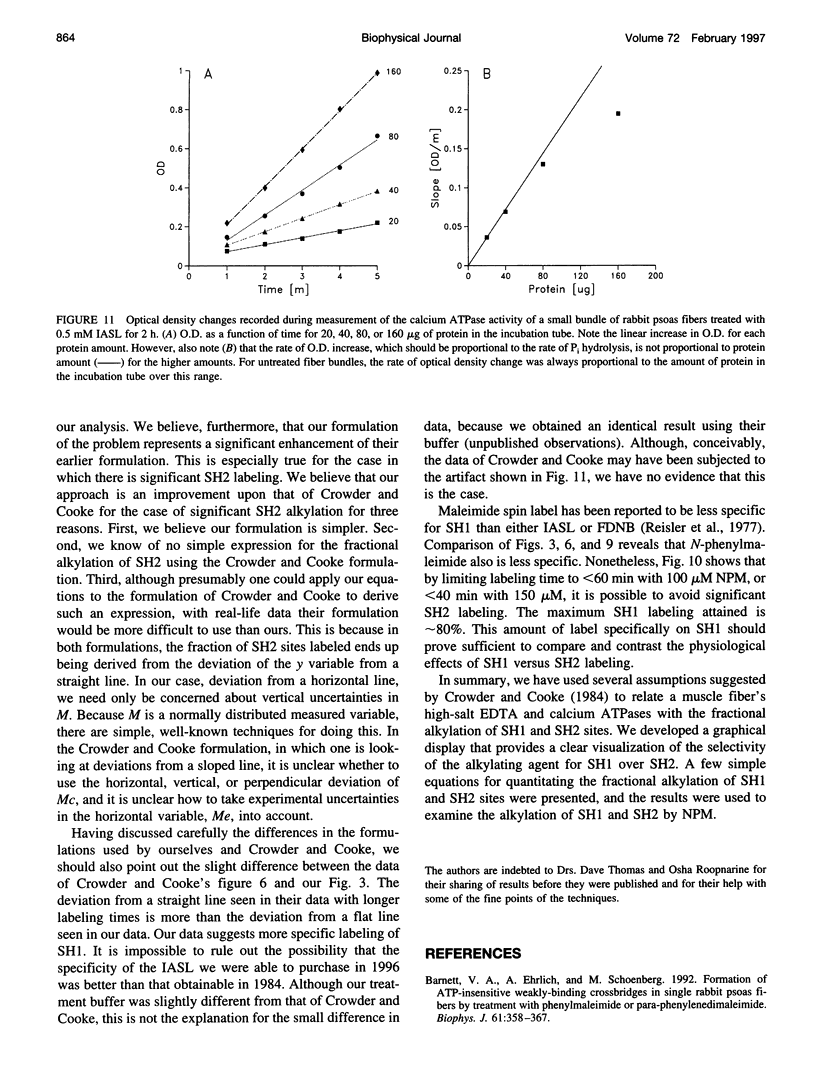
Previous assertions about the effect of alkylation of SH1 and SH2 on the myosin high-salt calcium and EDTA ATPases have been summarized, and a simple procedure for obtaining the fractional labeling of SH1 and SH2 after treatment of myosin with alkylating agents has been derived. A simple graphical procedure for illustrating the degree of preference of a particular alkylating agent for SH1 over SH2 has also been developed. The procedures we developed were validated by applying them to two previously studied compounds, 4-(2-iodoacetamido)-TEMPO and 2,4-dinitrofluorobenzine, and then were used to determine a procedure for maximizing the extent of labeling of SH1 alone by N-phenylmaleimide, a compound not previously studied in this manner. It was found that ∼80% of the SH1 sites could be alkylated without significant alkylation of SH2.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett V. A., Ehrlich A., Schoenberg M. Formation of ATP-insensitive weakly-binding crossbridges in single rabbit psoas fibers by treatment with phenylmaleimide or para-phenylenedimaleimide. Biophys J. 1992 Feb;61(2):358–367. doi: 10.1016/S0006-3495(92)81842-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaen S., Shimada M., Sugi H. Evidence for cooperative interactions of myosin heads with thin filament in the force generation of vertebrate skeletal muscle fibers. J Biol Chem. 1986 Oct 15;261(29):13632–13636. [PubMed] [Google Scholar]
- Crowder M. S., Cooke R. The effect of myosin sulphydryl modification on the mechanics of fibre contraction. J Muscle Res Cell Motil. 1984 Apr;5(2):131–146. doi: 10.1007/BF00712152. [DOI] [PubMed] [Google Scholar]
- Eastwood A. B., Wood D. S., Bock K. L., Sorenson M. M. Chemically skinned mammalian skeletal muscle. I. The structure of skinned rabbit psoas. Tissue Cell. 1979;11(3):553–566. doi: 10.1016/0040-8166(79)90062-4. [DOI] [PubMed] [Google Scholar]
- Ehrlich A., Barnett V. A., Chen H. C., Schoenberg M. The site and stoichiometry of the N-phenylmaleimide reaction with myosin when weakly-binding crossbridges are formed in skinned rabbit psoas fibers. Biochim Biophys Acta. 1995 Nov 21;1232(1-2):13–20. doi: 10.1016/0005-2728(95)00094-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lanzetta P. A., Alvarez L. J., Reinach P. S., Candia O. A. An improved assay for nanomole amounts of inorganic phosphate. Anal Biochem. 1979 Nov 15;100(1):95–97. doi: 10.1016/0003-2697(79)90115-5. [DOI] [PubMed] [Google Scholar]
- Reisler E., Burke M., Harrington W. F. Cooperative role of two sulfhydryl groups in myosin adenosine triphosphatase. Biochemistry. 1974 May 7;13(10):2014–2022. doi: 10.1021/bi00707a003. [DOI] [PubMed] [Google Scholar]
- Reisler E., Burke M., Harrington W. F. Reactivity of essential thiols of myosin. Chemical probes of the activated state. Biochemistry. 1977 Nov 29;16(24):5187–5191. doi: 10.1021/bi00643a005. [DOI] [PubMed] [Google Scholar]
- Reisler E. Sulfhydryl modification and labeling of myosin. Methods Enzymol. 1982;85(Pt B):84–93. doi: 10.1016/0076-6879(82)85012-x. [DOI] [PubMed] [Google Scholar]
- Roopnarine O., Hideg K., Thomas D. D. Saturation transfer electron parametric resonance of an indane-dione spin-label. Calibration with hemoglobin and application to myosin rotational dynamics. Biophys J. 1993 Jun;64(6):1896–1907. doi: 10.1016/S0006-3495(93)81561-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roopnarine O., Thomas D. D. A spin label that binds to myosin heads in muscle fibers with its principal axis parallel to the fiber axis. Biophys J. 1994 Oct;67(4):1634–1645. doi: 10.1016/S0006-3495(94)80636-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root D. D., Cheung P., Reisler E. Catalytic cooperativity induced by SH1 labeling of myosin filaments. Biochemistry. 1991 Jan 8;30(1):286–294. doi: 10.1021/bi00215a039. [DOI] [PubMed] [Google Scholar]
- Seidel J. C., Chopek M., Gergely J. Effect of nucleotides and pyrophosphate on spin labels bound to S1 thiol groups of myosin. Biochemistry. 1970 Aug 4;9(16):3265–3272. doi: 10.1021/bi00818a021. [DOI] [PubMed] [Google Scholar]
- Thomas D. D., Ishiwata S., Seidel J. C., Gergely J. Submillisecond rotational dynamics of spin-labeled myosin heads in myofibrils. Biophys J. 1980 Dec;32(3):873–889. doi: 10.1016/S0006-3495(80)85023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedner H., Wetzel R., Eckstein F. The nonessential nature of sulfhydryl groups for ATPase activity in myosin. A cyanylation study. J Biol Chem. 1978 Apr 25;253(8):2763–2768. [PubMed] [Google Scholar]
- Yamaguchi M., Sekine T. Sulfhydryl groups involved in the active site of myosin A adenosine triphosphatase. I. Specific blocking of the SH group responsible for the inhibitory phase in "B phasic response" of the catalytic activity. J Biochem. 1966 Jan;59(1):24–33. doi: 10.1093/oxfordjournals.jbchem.a128254. [DOI] [PubMed] [Google Scholar]


