Abstract
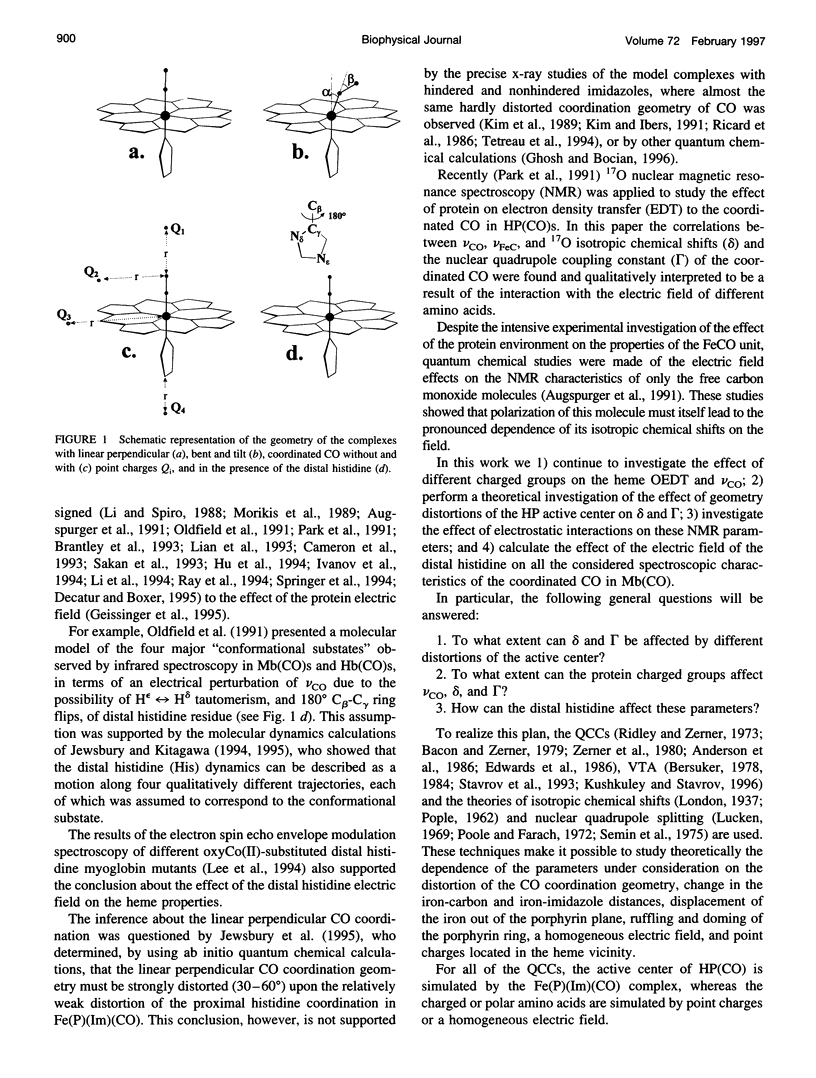
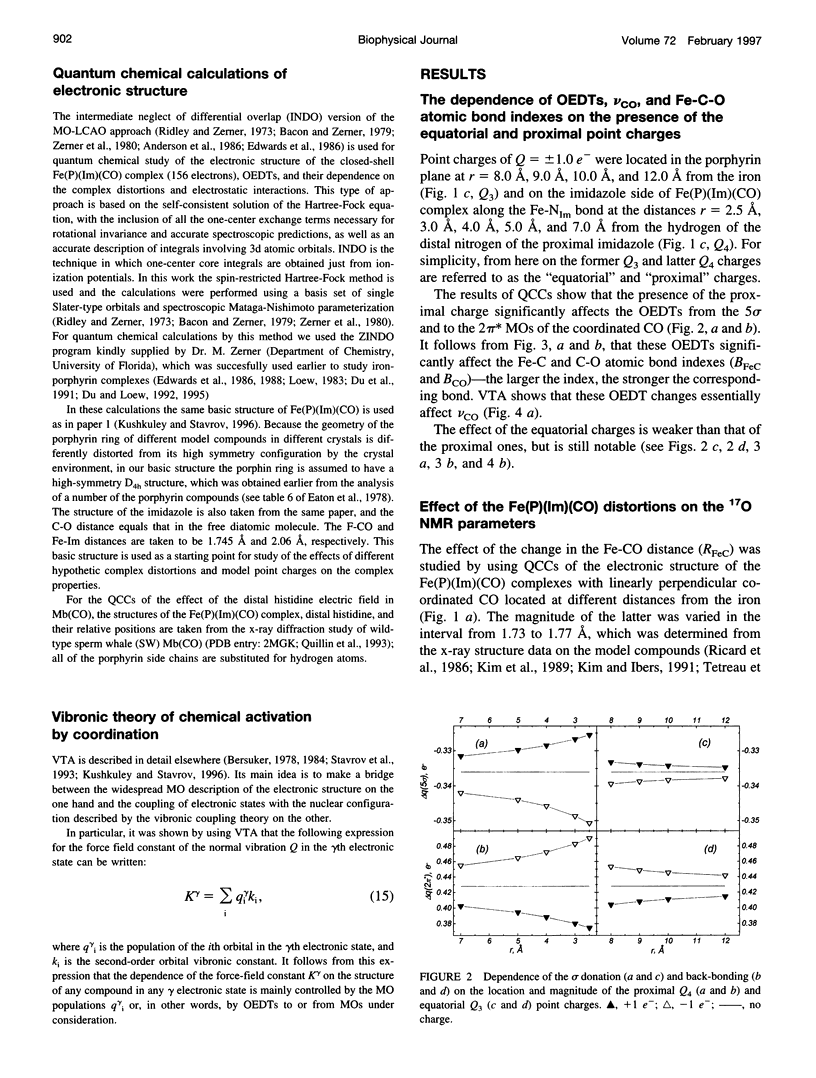
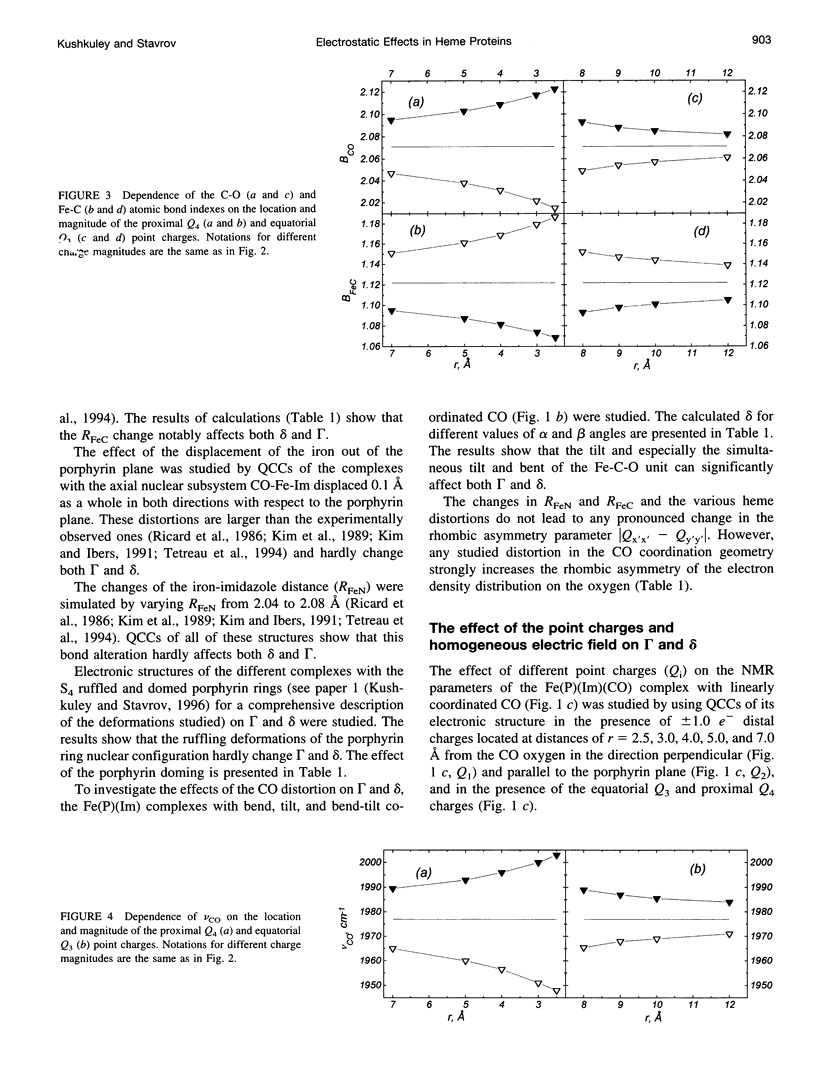
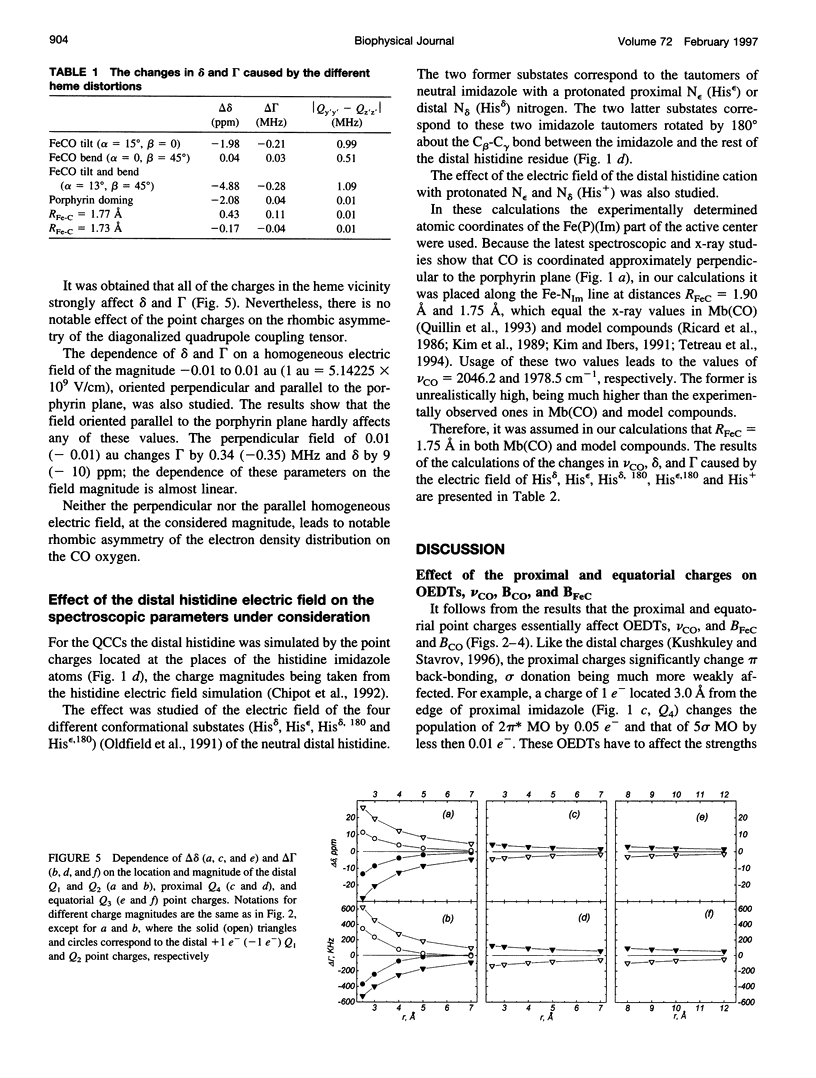
The quantum chemical calculations, vibronic theory of activation, and London-Pople approach are used to study the dependence of the C-O vibrational frequency, 17O isotropic chemical shift, and nuclear quadrupole coupling constant on the distortion of the porphyrin ring and geometry of the CO coordination, changes in the iron-carbon and iron-imidazole distances, magnitude of the iron displacement out of the porphyrin plane, and presence of the charged groups in the heme environment. It is shown that only the electrostatic interactions can cause the variation of all these parameters experimentally observed in different heme proteins, and the heme distortions could modulate this variation. The correlations between the theoretically calculated parameters are shown to be close to the experimentally observed ones. The study of the effect of the electric field of the distal histidine shows that the presence of the four C-O vibrational bands in the infrared absorption spectra of the carbon monoxide complexes of different myoglobins and hemoglobins can be caused by the different orientations of the different tautomeric forms of the distal histidine. The dependence of the 17O isotropic chemical shift and nuclear quadrupole coupling constant on pH and the distal histidine substitution can be also explained from the same point of view.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balasubramanian S., Lambright D. G., Boxer S. G. Perturbations of the distal heme pocket in human myoglobin mutants probed by infrared spectroscopy of bound CO: correlation with ligand binding kinetics. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4718–4722. doi: 10.1073/pnas.90.10.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantley R. E., Jr, Smerdon S. J., Wilkinson A. J., Singleton E. W., Olson J. S. The mechanism of autooxidation of myoglobin. J Biol Chem. 1993 Apr 5;268(10):6995–7010. [PubMed] [Google Scholar]
- Cameron A. D., Smerdon S. J., Wilkinson A. J., Habash J., Helliwell J. R., Li T., Olson J. S. Distal pocket polarity in ligand binding to myoglobin: deoxy and carbonmonoxy forms of a threonine68(E11) mutant investigated by X-ray crystallography and infrared spectroscopy. Biochemistry. 1993 Dec 7;32(48):13061–13070. doi: 10.1021/bi00211a016. [DOI] [PubMed] [Google Scholar]
- Coletta M., Ascenzi P., Traylor T. G., Brunori M. Kinetics of carbon monoxide binding to monomeric hemoproteins. Role of the proximal histidine. J Biol Chem. 1985 Apr 10;260(7):4151–4155. [PubMed] [Google Scholar]
- Decatur S. M., Boxer S. G. A test of the role of electrostatic interactions in determining the CO stretch frequency in carbonmonoxymyoglobin. Biochem Biophys Res Commun. 1995 Jul 6;212(1):159–164. doi: 10.1006/bbrc.1995.1950. [DOI] [PubMed] [Google Scholar]
- Du P., Loew G. H. Theoretical study of model compound I complexes of horseradish peroxidase and catalase. Biophys J. 1995 Jan;68(1):69–80. doi: 10.1016/S0006-3495(95)80160-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchsman W. H., Appleby C. A. CO and O2 complexes of soybean leghemoglobins: pH effects upon infrared and visible spectra. Comparisons with CO and O2 complexes of myoglobin and hemoglobin. Biochemistry. 1979 Apr 3;18(7):1309–1321. doi: 10.1021/bi00574a030. [DOI] [PubMed] [Google Scholar]
- Giacometti G. M., Traylor T. G., Ascenzi P., Brunori M., Antonini E. Reactivity of ferrous myoglobin at low pH. J Biol Chem. 1977 Nov 10;252(21):7447–7448. [PubMed] [Google Scholar]
- Jewsbury P., Kitagawa T. Distal residue-CO interaction in carbonmonoxy myoglobins: a molecular dynamics study of three distal mutants. Biophys J. 1995 Apr;68(4):1283–1294. doi: 10.1016/S0006-3495(95)80302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewsbury P., Kitagawa T. The distal residue-CO interaction in carbonmonoxy myoglobins: a molecular dynamics study of two distal histidine tautomers. Biophys J. 1994 Dec;67(6):2236–2250. doi: 10.1016/S0006-3495(94)80708-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushkuley B., Stavrov S. S. Theoretical study of the distal-side steric and electrostatic effects on the vibrational characteristics of the FeCO unit of the carbonylheme proteins and their models. Biophys J. 1996 Mar;70(3):1214–1229. doi: 10.1016/S0006-3495(96)79680-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. C., Ikeda-Saito M., Yonetani T., Magliozzo R. S., Peisach J. Hydrogen bonding to the bound dioxygen in oxy cobaltous myoglobin reduces the superhyperfine coupling to the proximal histidine. Biochemistry. 1992 Aug 18;31(32):7274–7281. doi: 10.1021/bi00147a010. [DOI] [PubMed] [Google Scholar]
- Lee H. C., Peisach J., Dou Y., Ikeda-Saito M. Electron-nuclear coupling to the proximal histidine in oxy cobalt-substituted distal histidine mutants of human myoglobin. Biochemistry. 1994 Jun 21;33(24):7609–7618. doi: 10.1021/bi00190a014. [DOI] [PubMed] [Google Scholar]
- Lee H. C., Peisach J., Tsuneshige A., Yonetani T. Electron spin echo envelope modulation study of oxygenated iron-cobalt hybrid hemoglobins reveals molecular features analogous to those of the oxy ferrous protein. Biochemistry. 1995 May 23;34(20):6883–6891. doi: 10.1021/bi00020a036. [DOI] [PubMed] [Google Scholar]
- Lee H. C., Wittenberg J. B., Peisach J. Role of hydrogen bonding to bound dioxygen in soybean leghemoglobin. Biochemistry. 1993 Nov 2;32(43):11500–11506. doi: 10.1021/bi00094a005. [DOI] [PubMed] [Google Scholar]
- Li T., Quillin M. L., Phillips G. N., Jr, Olson J. S. Structural determinants of the stretching frequency of CO bound to myoglobin. Biochemistry. 1994 Feb 15;33(6):1433–1446. doi: 10.1021/bi00172a021. [DOI] [PubMed] [Google Scholar]
- Lian T., Locke B., Kitagawa T., Nagai M., Hochstrasser R. M. Determination of Fe-CO geometry in the subunits of carbonmonoxy hemoglobin M Boston using femtosecond infrared spectroscopy. Biochemistry. 1993 Jun 8;32(22):5809–5814. doi: 10.1021/bi00073a013. [DOI] [PubMed] [Google Scholar]
- Lim M., Jackson T. A., Anfinrud P. A. Binding of CO to myoglobin from a heme pocket docking site to form nearly linear Fe-C-O. Science. 1995 Aug 18;269(5226):962–966. doi: 10.1126/science.7638619. [DOI] [PubMed] [Google Scholar]
- Ling J., Li T., Olson J. S., Bocian D. F. Identification of the iron-carbonyl stretch in distal histidine mutants of carbonmonoxymyoglobin. Biochim Biophys Acta. 1994 Dec 30;1188(3):417–421. doi: 10.1016/0005-2728(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Morikis D., Champion P. M., Springer B. A., Sligar S. G. Resonance raman investigations of site-directed mutants of myoglobin: effects of distal histidine replacement. Biochemistry. 1989 May 30;28(11):4791–4800. doi: 10.1021/bi00437a041. [DOI] [PubMed] [Google Scholar]
- Parak F., Knapp E. W. A consistent picture of protein dynamics. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7088–7092. doi: 10.1073/pnas.81.22.7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K. D., Guo K. M., Adebodun F., Chiu M. L., Sligar S. G., Oldfield E. Distal and proximal ligand interactions in heme proteins: correlations between C-O and Fe-C vibrational frequencies, oxygen-17 and carbon-13 nuclear magnetic resonance chemical shifts, and oxygen-17 nuclear quadrupole coupling constants in C17O- and 13CO-labeled species. Biochemistry. 1991 Mar 5;30(9):2333–2347. doi: 10.1021/bi00223a007. [DOI] [PubMed] [Google Scholar]
- Potter W. T., Hazzard J. H., Choc M. G., Tucker M. P., Caughey W. S. Infrared spectra of carbonyl hemoglobins: characterization of dynamic heme pocket conformers. Biochemistry. 1990 Jul 3;29(26):6283–6295. doi: 10.1021/bi00478a025. [DOI] [PubMed] [Google Scholar]
- Quillin M. L., Arduini R. M., Olson J. S., Phillips G. N., Jr High-resolution crystal structures of distal histidine mutants of sperm whale myoglobin. J Mol Biol. 1993 Nov 5;234(1):140–155. doi: 10.1006/jmbi.1993.1569. [DOI] [PubMed] [Google Scholar]
- Quillin M. L., Li T., Olson J. S., Phillips G. N., Jr, Dou Y., Ikeda-Saito M., Regan R., Carlson M., Gibson Q. H., Li H. Structural and functional effects of apolar mutations of the distal valine in myoglobin. J Mol Biol. 1995 Jan 27;245(4):416–436. doi: 10.1006/jmbi.1994.0034. [DOI] [PubMed] [Google Scholar]
- Ramsden J., Spiro T. G. Resonance Raman evidence that distal histidine protonation removes the steric hindrance to upright binding of carbon monoxide by myoglobin. Biochemistry. 1989 Apr 18;28(8):3125–3128. doi: 10.1021/bi00434a001. [DOI] [PubMed] [Google Scholar]
- Sage J. T., Morikis D., Champion P. M. Spectroscopic studies of myoglobin at low pH: heme structure and ligation. Biochemistry. 1991 Feb 5;30(5):1227–1237. doi: 10.1021/bi00219a010. [DOI] [PubMed] [Google Scholar]
- Sakan Y., Ogura T., Kitagawa T., Fraunfelter F. A., Mattera R., Ikeda-Saito M. Time-resolved resonance Raman study on the binding of carbon monoxide to recombinant human myoglobin and its distal histidine mutants. Biochemistry. 1993 Jun 8;32(22):5815–5824. doi: 10.1021/bi00073a014. [DOI] [PubMed] [Google Scholar]
- Traylor T. G., Deardurff L. A., Coletta M., Ascenzi P., Antonini E., Brunori M. Reactivity of ferrous heme proteins at low pH. J Biol Chem. 1983 Oct 25;258(20):12147–12148. [PubMed] [Google Scholar]
- Ullrich V. Cytochrome P450 and biological hydroxylation reactions. Top Curr Chem. 1979;83:67–104. doi: 10.1007/BFb0019663. [DOI] [PubMed] [Google Scholar]
- Yang F., Phillips G. N., Jr Crystal structures of CO-, deoxy- and met-myoglobins at various pH values. J Mol Biol. 1996 Mar 8;256(4):762–774. doi: 10.1006/jmbi.1996.0123. [DOI] [PubMed] [Google Scholar]
- Zhu L., Sage J. T., Rigos A. A., Morikis D., Champion P. M. Conformational interconversion in protein crystals. J Mol Biol. 1992 Mar 5;224(1):207–215. doi: 10.1016/0022-2836(92)90584-7. [DOI] [PubMed] [Google Scholar]


