Abstract
Treatment of ox and dog thyroid slices in vitro with either thyrotropin or dibutyryl cyclic AMP elicited a variety of changes in polyribosome distribution. The most marked and consistent responses were decreases in both free and membrane-bound monoribosomes with a concomitant increase in the specific peak of thyroglobulin-synthesizing polyribosomes. On some occasions there was a shift towards heavier aggregates in the free polyribosomes. The increase in the amount of thyroglobulin-synthesizing polyribosomes was not accompanied by a shift in its location on the gradients. These changes were apparent within 30 min of thyrotropin addition and within 60 min of the addition of dibutyryl cyclic AMP. It is suggested that the major initial effect on translation of both thyrotropin and dibutyryl cyclic AMP is to stimulate the recruitment of pre-existing free monoribosomes on to pre-existing unloaded or under-loaded thyroglobulin mRNA molecules.
Full text
PDF
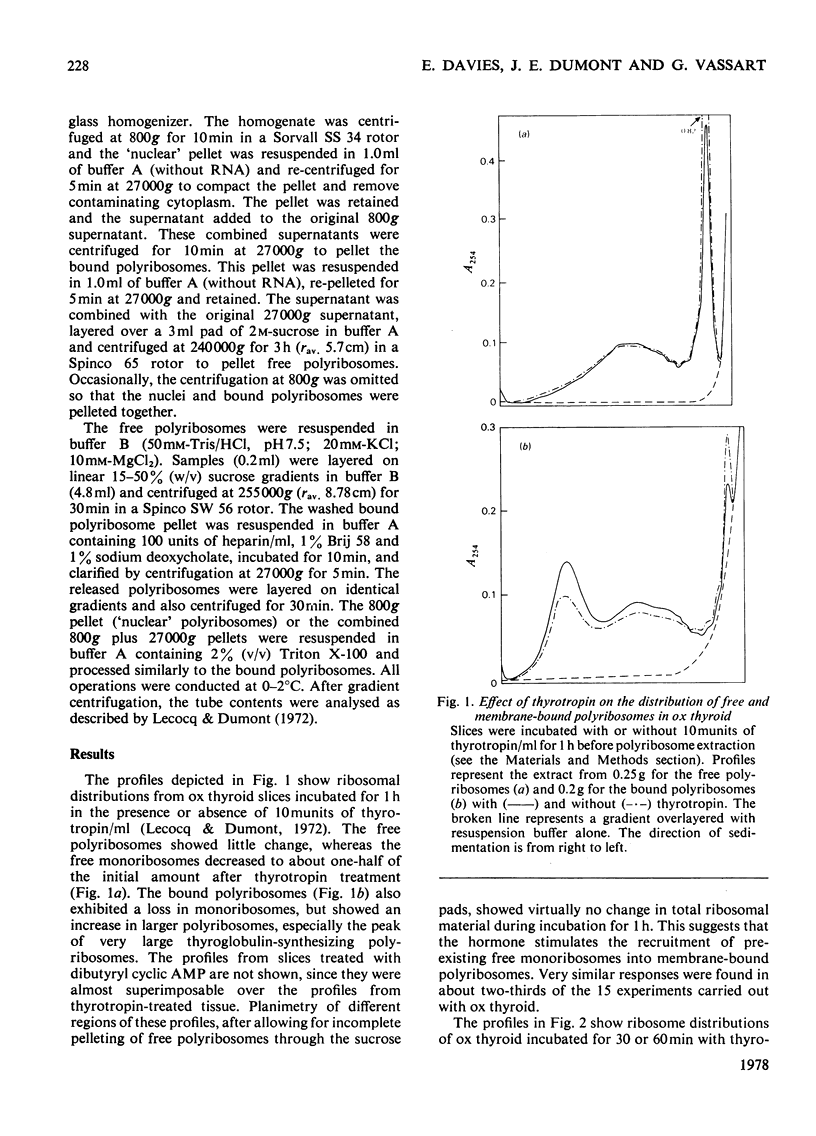
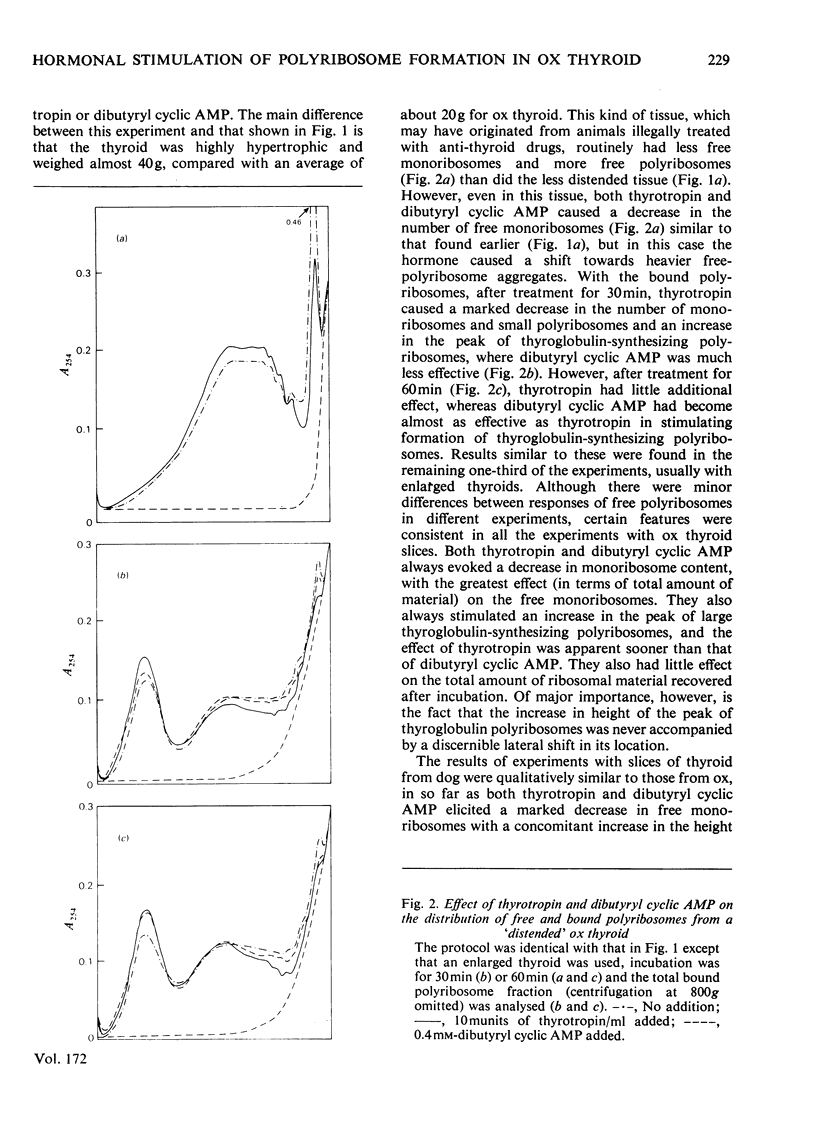


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G., Potter V. R. Studies on free and membrane-bound ribosomes in rat liver. II. Interaction of ribosomes and membranes. J Mol Biol. 1967 Jun 14;26(2):293–301. doi: 10.1016/0022-2836(67)90298-7. [DOI] [PubMed] [Google Scholar]
- Davies E., Dumont J. E., Vassart G. Improved techniques for the isolation of intact thyroglobulin-synthesizing polysomes. Anal Biochem. 1977 May 15;80(1):289–297. doi: 10.1016/0003-2697(77)90647-9. [DOI] [PubMed] [Google Scholar]
- Davies E. Polyribosomes from Peas: VI. Auxin-stimulated Recruitment of Free Monosomes into Membrane-bound Polysomes. Plant Physiol. 1976 Apr;57(4):516–518. doi: 10.1104/pp.57.4.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Nayer P., Labrique M., De Visscher M. Synthesis and association of thyroglobulin subunits in a polyribosomal cell-free system. Biochim Biophys Acta. 1973 Jan 19;294(1):309–321. [PubMed] [Google Scholar]
- Dumont J. E. The action of thyrotropin on thyroid metabolism. Vitam Horm. 1971;29:287–412. doi: 10.1016/s0083-6729(08)60051-5. [DOI] [PubMed] [Google Scholar]
- Lecocq R. E., Dumont J. E. In vivo and in vitro effects of thyrotropin on ribosomal pattern of dog thyroid. Biochim Biophys Acta. 1973 Mar 19;299(2):304–311. doi: 10.1016/0005-2787(73)90354-7. [DOI] [PubMed] [Google Scholar]
- Lecocq R. E., Dumont J. E. Stimulation by thyrotropin of amino acid incorporation into proteins in dog thyroid slices in vitro. Biochim Biophys Acta. 1972 Oct 27;281(3):434–441. doi: 10.1016/0005-2787(72)90459-5. [DOI] [PubMed] [Google Scholar]
- Lissitzky S., Manté S., Attali J. C., Cartouzou G. Action of 3',5'-cyclic adenosinemonophosphate on the protein synthesizing capacity of thyroid polyribosomes in vitro. Biochem Biophys Res Commun. 1969 May 22;35(4):437–443. doi: 10.1016/0006-291x(69)90364-7. [DOI] [PubMed] [Google Scholar]
- Marbaix G., Huez G., Nokin P., Cleuter Y. Free cytoplasmic alpha-globin messenger RNA appears during the maturation of rabbit reticulocytes. FEBS Lett. 1976 Jul 15;66(2):269–273. doi: 10.1016/0014-5793(76)80520-0. [DOI] [PubMed] [Google Scholar]
- McKnight G. S., Pennequin P., Schimke R. T. Induction of ovalbumin mRNA sequences by estrogen and progesterone in chick oviduct as measured by hybridization to complementary DNA. J Biol Chem. 1975 Oct 25;250(20):8105–8110. [PubMed] [Google Scholar]
- Pavlovic-Hournac M., Rappaport L., Nunez J. Incorporation of labeled amino acid into protein by thyroid glands from hypophysectomized rats. I. In vitro studies. Endocrinology. 1971 Dec;89(6):1477–1484. doi: 10.1210/endo-89-6-1477. [DOI] [PubMed] [Google Scholar]
- Pavlović-Hournac M., Delbauffe D. Protein metabolism in hypo- and hyperstimulated rat thyroid glands. II. Degradation of newly formed thyroidal proteins. Horm Metab Res. 1976 Jan;8(1):55–61. doi: 10.1055/s-0028-1093673. [DOI] [PubMed] [Google Scholar]
- Rottman F., Shatkin A. J., Perry R. P. Sequences containing methylated nucleotides at the 5' termini of messenger RNAs: possible implications for processing. Cell. 1974 Nov;3(3):197–199. doi: 10.1016/0092-8674(74)90131-7. [DOI] [PubMed] [Google Scholar]
- SEED R. W., GOLDBERG I. H. BIOSYNTHESIS OF THYROGLOBULIN. II. ROLE OF SUBUNITS, IODINATION, AND RIBONUCLEIC ACID SYNTHESIS. J Biol Chem. 1965 Feb;240:764–773. [PubMed] [Google Scholar]
- Salabé H., D'Armiento M., Salabé G. B., Andreoli M., Blecher M. Cyclic AMP and thyroid hormone synthesis. I. Stimulation of the biosynthesis of thyroglobulin-related polypeptides and proteins in bovine thyroid slices. Horm Res. 1973;4(5):274–287. doi: 10.1159/000178314. [DOI] [PubMed] [Google Scholar]
- Sherwin J. R., Tong W. Stimulatory actions of thyrotropin and dibutyryl cyclic AMP on transcription and translation in the regulation of thyroidal protein synthesis. Biochim Biophys Acta. 1976 Apr 2;425(4):502–510. doi: 10.1016/0005-2787(76)90014-9. [DOI] [PubMed] [Google Scholar]
- Vassart G. M., Dumont J. E., Cantraine F. R. Simulation of polyribosome disaggregation. Biochim Biophys Acta. 1970 Nov 12;224(1):155–164. doi: 10.1016/0005-2787(70)90629-5. [DOI] [PubMed] [Google Scholar]
- Vassart G., Dumont J. E., Cantraine F. R. Translational control of protein synthesis: a simulation study. Biochim Biophys Acta. 1971 Oct;247(3):471–485. doi: 10.1016/0005-2787(71)90034-7. [DOI] [PubMed] [Google Scholar]
- Vassart G., Dumont J. E. Identification of polysomes synthesizing thyroglobulin. Eur J Biochem. 1973 Jan 15;32(2):322–330. doi: 10.1111/j.1432-1033.1973.tb02613.x. [DOI] [PubMed] [Google Scholar]
- Wägar G., Ekholm R., Björkman U. Action of thyrotrophin (TSH) on thyroid protein synthesis in vivo and in vitro. Acta Endocrinol (Copenh) 1973 Mar;72(3):453–463. doi: 10.1530/acta.0.0720453. [DOI] [PubMed] [Google Scholar]


