Abstract
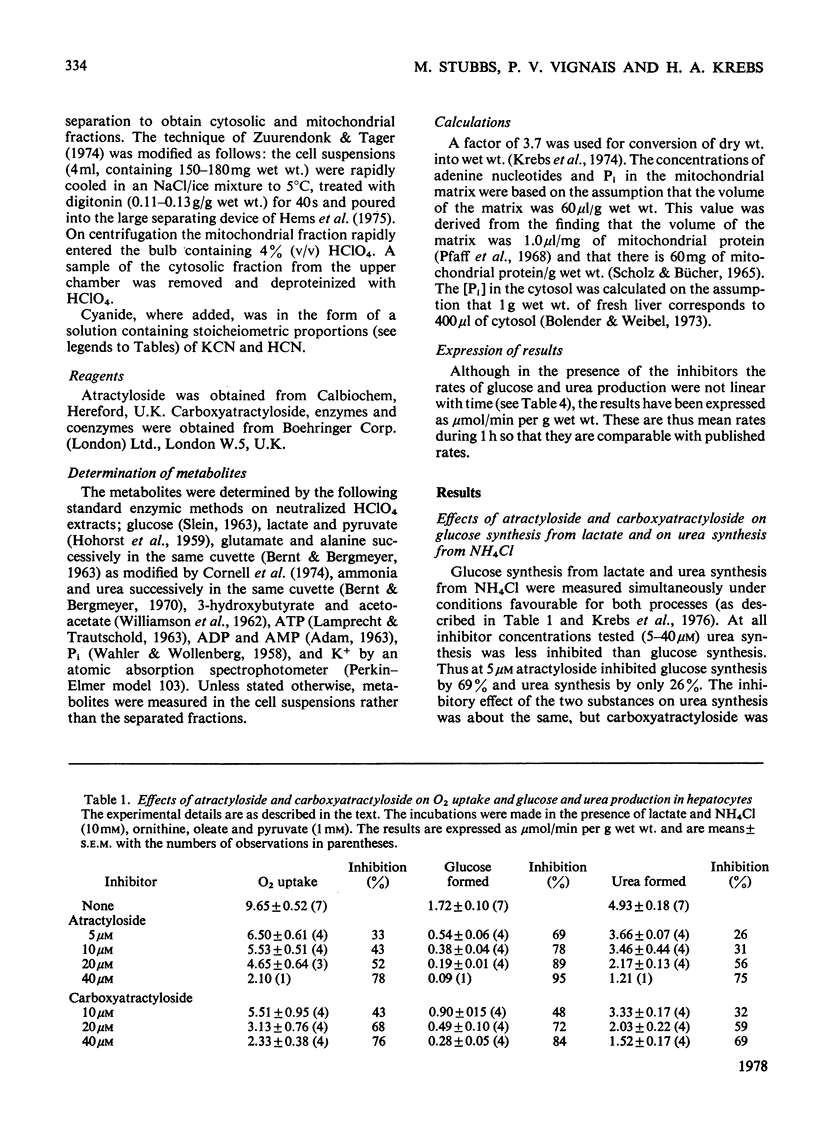
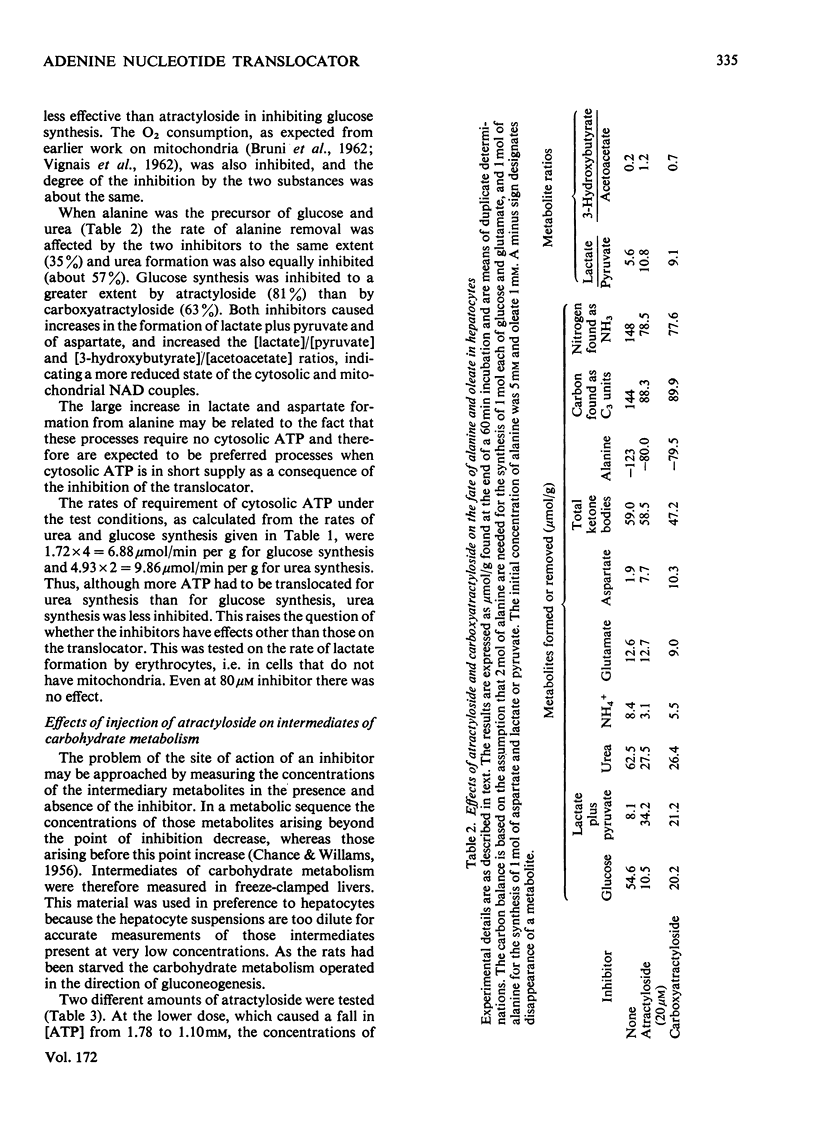
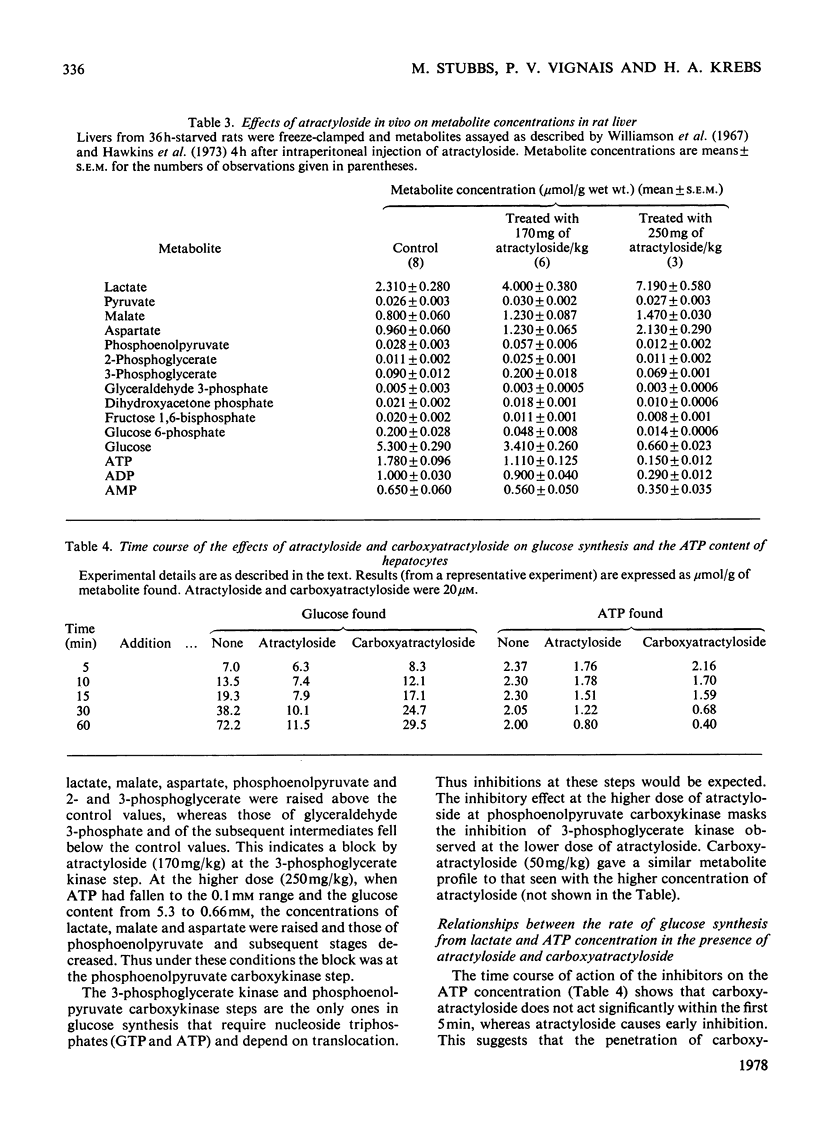
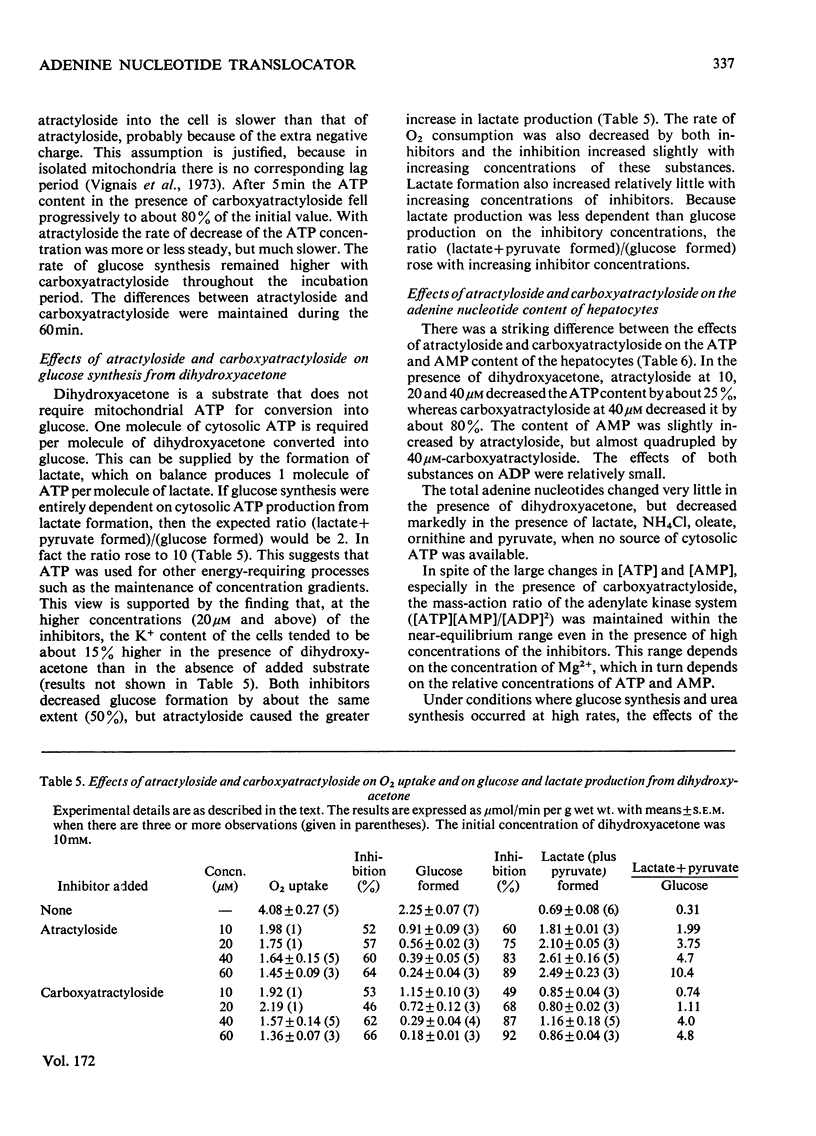
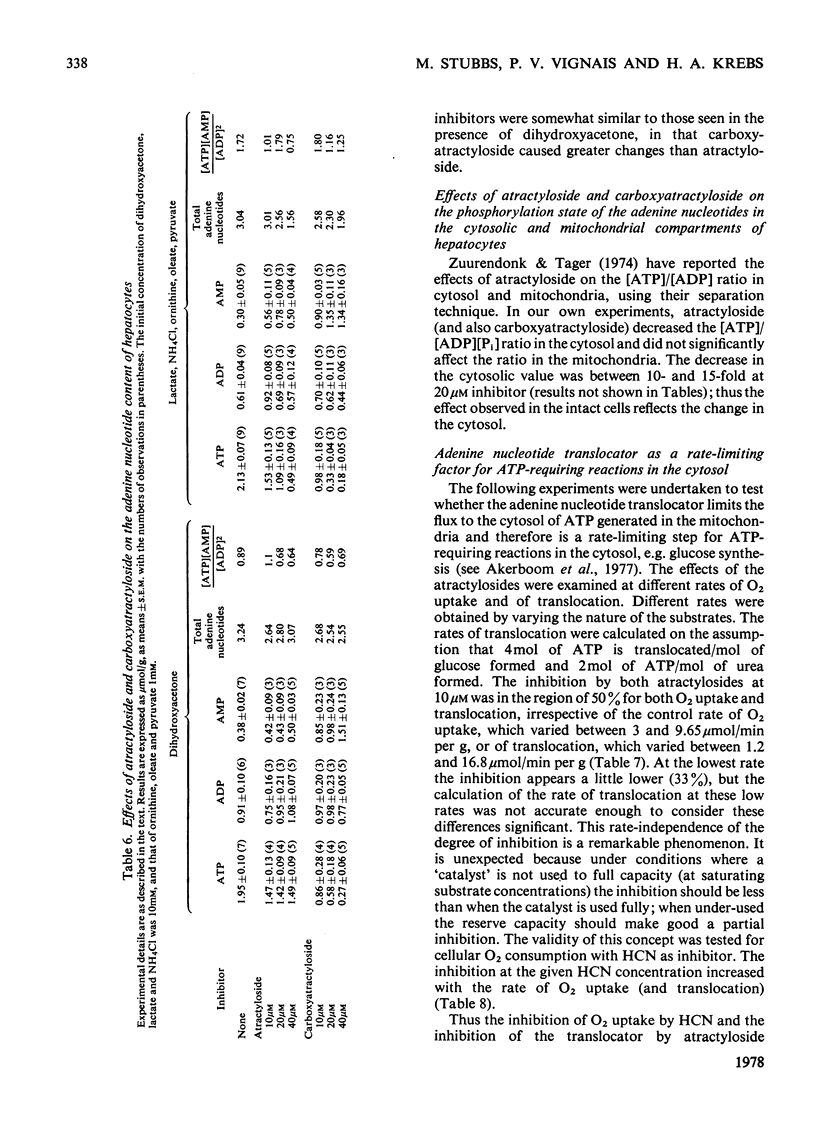
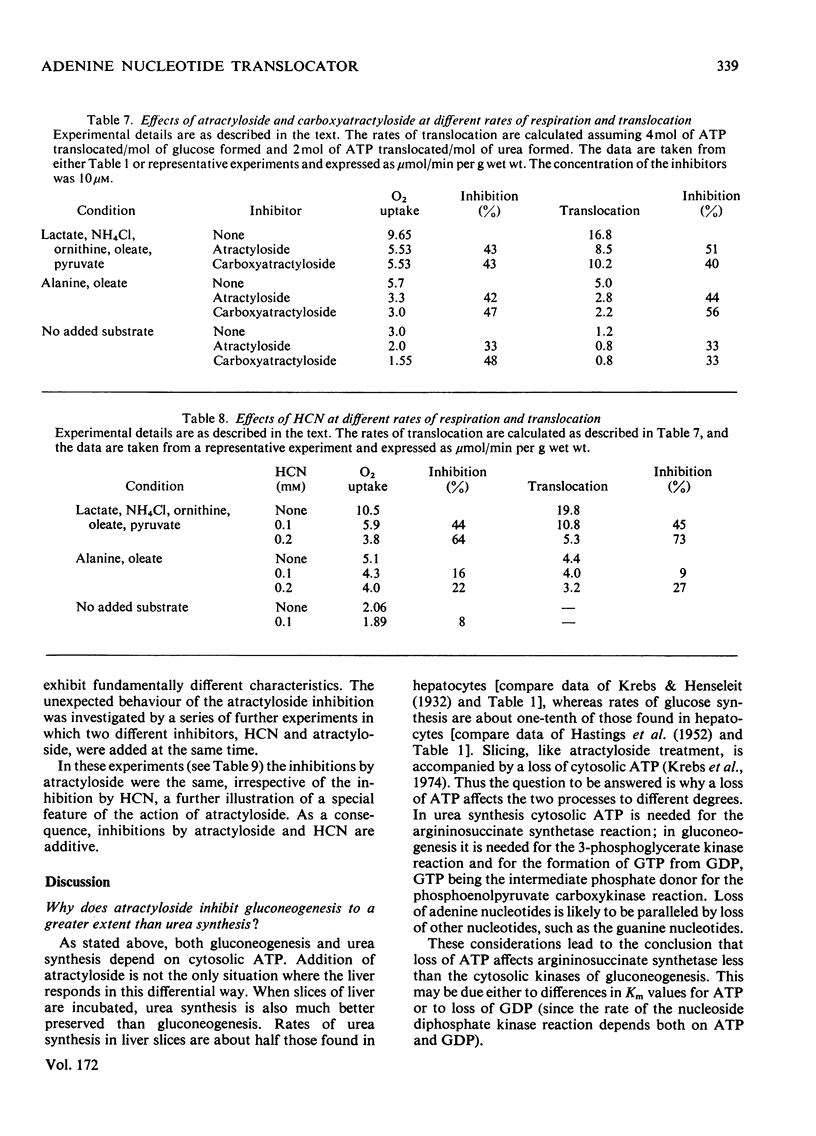
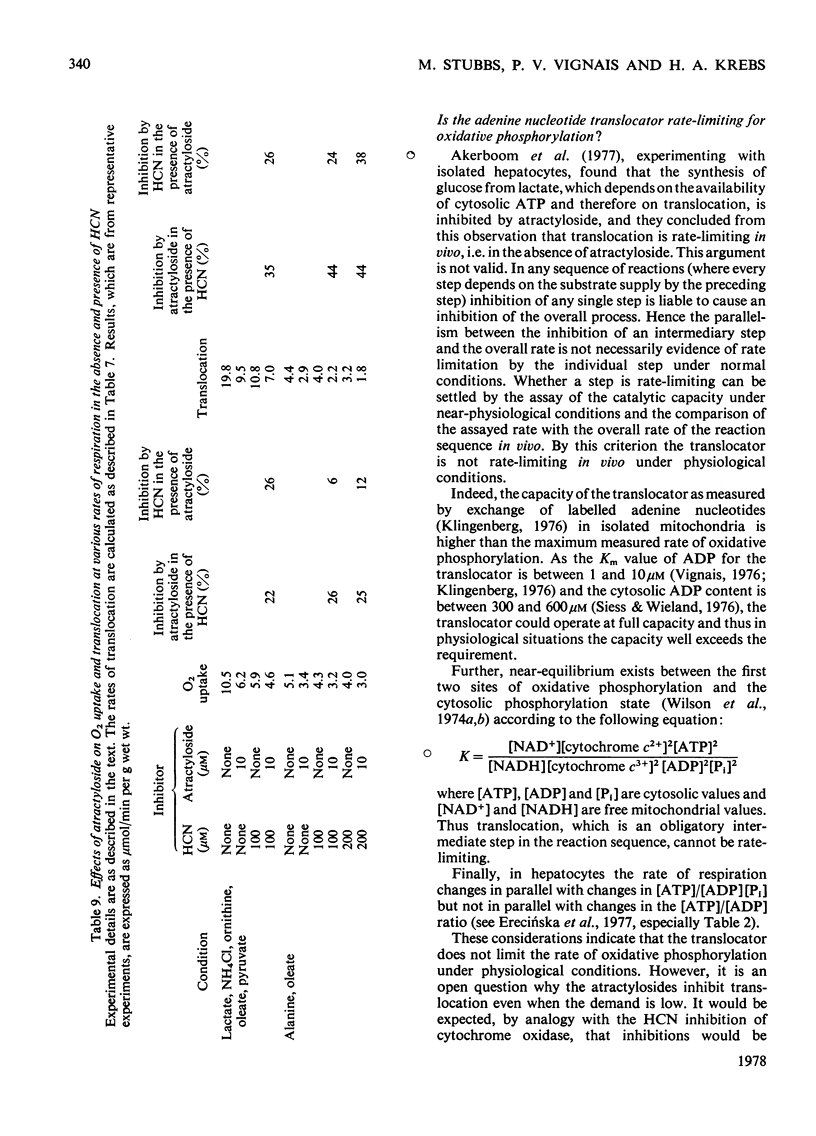
1. The effects of atractyloside and carboxyatractyloside (between 5 and 40μm) on O2 uptake, glucose synthesis, urea synthesis, the adenine nucleotide content and the intracellular K+ concentration were measured in isolated hepatocytes. 2. Urea synthesis was much less inhibited than glucose synthesis by both atractylosides. Measurements of intermediary metabolites of carbohydrate metabolism in freeze-clamped liver after injection of atractyloside into rats indicate that inhibition of gluconeogenesis is due to interference at the cytosolic reactions requiring ATP (phosphoenolpyruvate carboxykinase and 3-phosphoglycerate kinase). 3. The decrease in [ATP]/[ADP]×[Pi] after addition of atractyloside or carboxyatractyloside was restricted to the cytosol. 4. Dihydroxyacetone can be converted either into glucose with the consumption of 2mol of ATP (per mol of glucose) or into lactate with the production of 2mol of ATP. In the presence of high concentrations of atractyloside and carboxyatractyloside more ATP was produced than was used for the synthesis of glucose from dihydroxyacetone, probably for the maintenance of intracellular [K+]. 5. When the rates of respiration were altered by changing substrates, the degrees of inhibition of respiration and translocation by a given concentration of the atractylosides were the same, whereas at a given concentration of HCN the degree of inhibition was high at higher initial rates, and low at lower initial rates. 6. Inhibition of a complex series of reactions by atractyloside does not necessarily indicate that the translocator is a rate-limiting step in that sequence as Th. P. M. Akerboom, H. Bookelman & J. M. Tager [(1977) FEBS. Lett. 74, 50–54] assume. This point is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerboom T. P., Bookelman H., Tager J. M. Control of ATP transport across the mitochondrial membrane in isolated rat-liver cells. FEBS Lett. 1977 Feb 15;74(1):50–54. doi: 10.1016/0014-5793(77)80750-3. [DOI] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolender R. P., Weibel E. R. A morphometric study of the removal of phenobarbital-induced membranes from hepatocytes after cessation of threatment. J Cell Biol. 1973 Mar;56(3):746–761. doi: 10.1083/jcb.56.3.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Cornell N. W., Lund P., Krebs H. A. The effect of lysine on gluconeogenesis from lactate in rat hepatocytes. Biochem J. 1974 Aug;142(2):327–337. doi: 10.1042/bj1420327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erecińska M., Stubbs M., Miyata Y., Ditre C. M. Regulation of cellular metabolism by intracellular phosphate. Biochim Biophys Acta. 1977 Oct 12;462(1):20–35. doi: 10.1016/0005-2728(77)90186-4. [DOI] [PubMed] [Google Scholar]
- HASTINGS A. B., TENG C. T., NESBETT F. B., SINEX F. M. Studies on carbohydrate metabolism in rat liver slices. I. The effect of cations in the media. J Biol Chem. 1952 Jan;194(1):69–81. [PubMed] [Google Scholar]
- HOHORST H. J., KREUTZ F. H., BUECHER T. [On the metabolite content and the metabolite concentration in the liver of the rat]. Biochem Z. 1959;332:18–46. [PubMed] [Google Scholar]
- Hawkins R. A., Houghton C. R., Williamson D. H. Hepatic redox state and gluconeogenesis from lactate in vivo in the rat. Biochem J. 1973 Jan;132(1):19–25. doi: 10.1042/bj1320019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt H. W., Pfaff E. Adenine nucleotide translocation in mitochondria. Quantitative evaluation of the correlation between the phosphorylation of endogenous and exogenous ADP in mitochondria. Eur J Biochem. 1969 Oct;10(3):494–500. doi: 10.1111/j.1432-1033.1969.tb00716.x. [DOI] [PubMed] [Google Scholar]
- Hems R., Lund P., Krebs H. A. Rapid separation of isolated hepatocytes or similar tissue fragments for analysis of cell constituents. Biochem J. 1975 Jul;150(1):47–50. doi: 10.1042/bj1500047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp A., Jr, Groot G. S., Reitsma H. J. Oxidative phosphorylation as a function of temperature. Biochim Biophys Acta. 1969 May;180(1):28–34. doi: 10.1016/0005-2728(69)90190-x. [DOI] [PubMed] [Google Scholar]
- Klingenberg M. Mitochondria metabolite transport. FEBS Lett. 1970 Feb 16;6(3):145–154. doi: 10.1016/0014-5793(70)80044-8. [DOI] [PubMed] [Google Scholar]
- Pfaff E., Klingenberg M., Ritt E., Vogell W. Korrelation des unspezifisch permeablen mitochondrialen Raumes mit dem "Intermembran-Raum". Eur J Biochem. 1968 Jul;5(2):222–232. doi: 10.1111/j.1432-1033.1968.tb00361.x. [DOI] [PubMed] [Google Scholar]
- Siess E. A., Wieland O. H. Phosphorylation state of cytosolic and mitochondrial adenine nucleotides and of pyruvate dehydrogenase in isolated rat liver cells. Biochem J. 1976 Apr 15;156(1):91–102. doi: 10.1042/bj1560091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VIGNAIS P. V., VIGNAIS P. M., STANISLAS E. Action of potassium atractylate on oxidative phosphorylation in mitochondria and in submitochondrial particles. Biochim Biophys Acta. 1962 Jul 2;60:284–300. doi: 10.1016/0006-3002(62)90404-3. [DOI] [PubMed] [Google Scholar]
- Vignais P. V. Molecular and physiological aspects of adenine nucleotide transport in mitochondria. Biochim Biophys Acta. 1976 Apr 30;456(1):1–38. doi: 10.1016/0304-4173(76)90007-0. [DOI] [PubMed] [Google Scholar]
- Vignais P. V., Vignais P. M., Defaye G. Adenosine diphosphate translocation in mitochondria. Nature of the receptor site for carboxyatractyloside (gummiferin). Biochemistry. 1973 Apr 10;12(8):1508–1519. doi: 10.1021/bi00732a007. [DOI] [PubMed] [Google Scholar]
- WAHLER B. E., WOLLENBERGER A. Zur Bestimmung des Orthophosphats neben säure-molybdat-labilen Phosphorsäureverbindungen. Biochem Z. 1958;329(6):508–520. [PubMed] [Google Scholar]
- WILLIAMSON D. H., MELLANBY J., KREBS H. A. Enzymic determination of D(-)-beta-hydroxybutyric acid and acetoacetic acid in blood. Biochem J. 1962 Jan;82:90–96. doi: 10.1042/bj0820090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., Lund P., Krebs H. A. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J. 1967 May;103(2):514–527. doi: 10.1042/bj1030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. F., Stubbs M., Oshino N., Erecińska M. Thermodynamic relationships between the mitochondrial oxidation-reduction reactions and cellular ATP levels in ascites tumor cells and perfused rat liver. Biochemistry. 1974 Dec 17;13(26):5305–5311. doi: 10.1021/bi00723a008. [DOI] [PubMed] [Google Scholar]
- Wilson D. F., Stubbs M., Veech R. L., Erecińska M., Krebs H. A. Equilibrium relations between the oxidation-reduction reactions and the adenosine triphosphate synthesis in suspensions of isolated liver cells. Biochem J. 1974 Apr;140(1):57–64. doi: 10.1042/bj1400057. [DOI] [PMC free article] [PubMed] [Google Scholar]


