Abstract
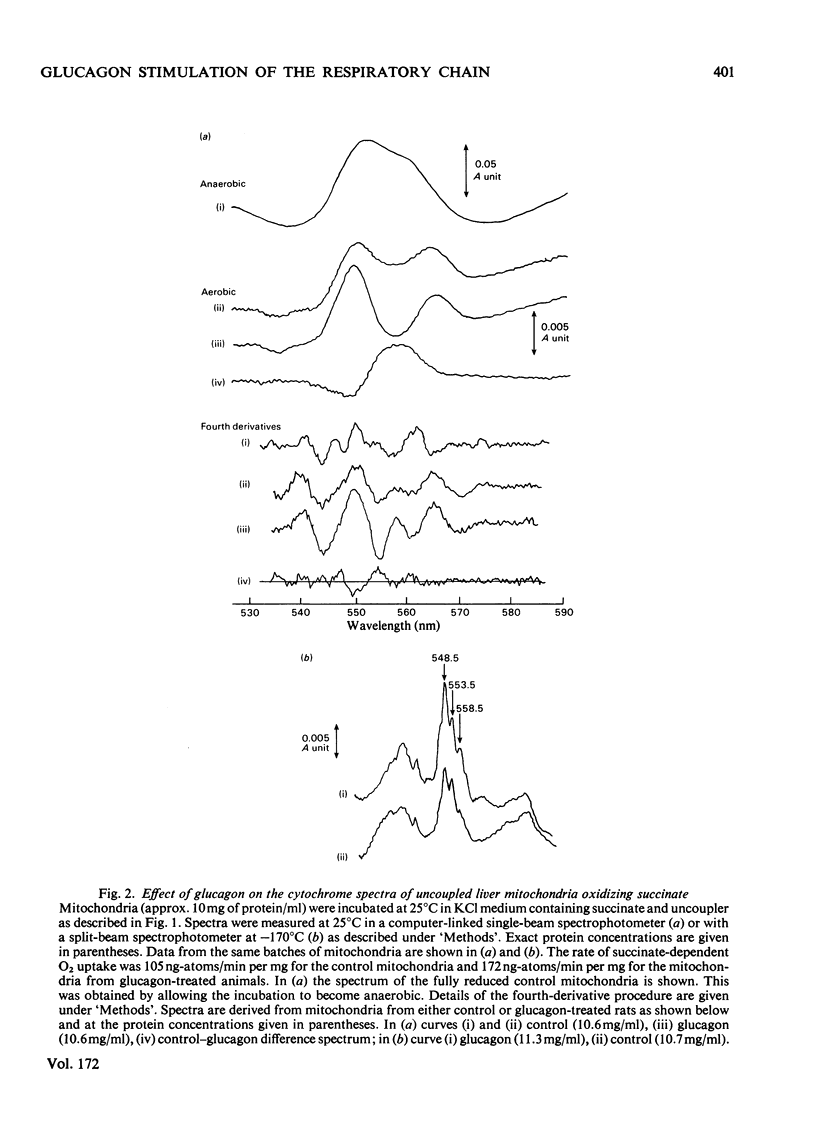
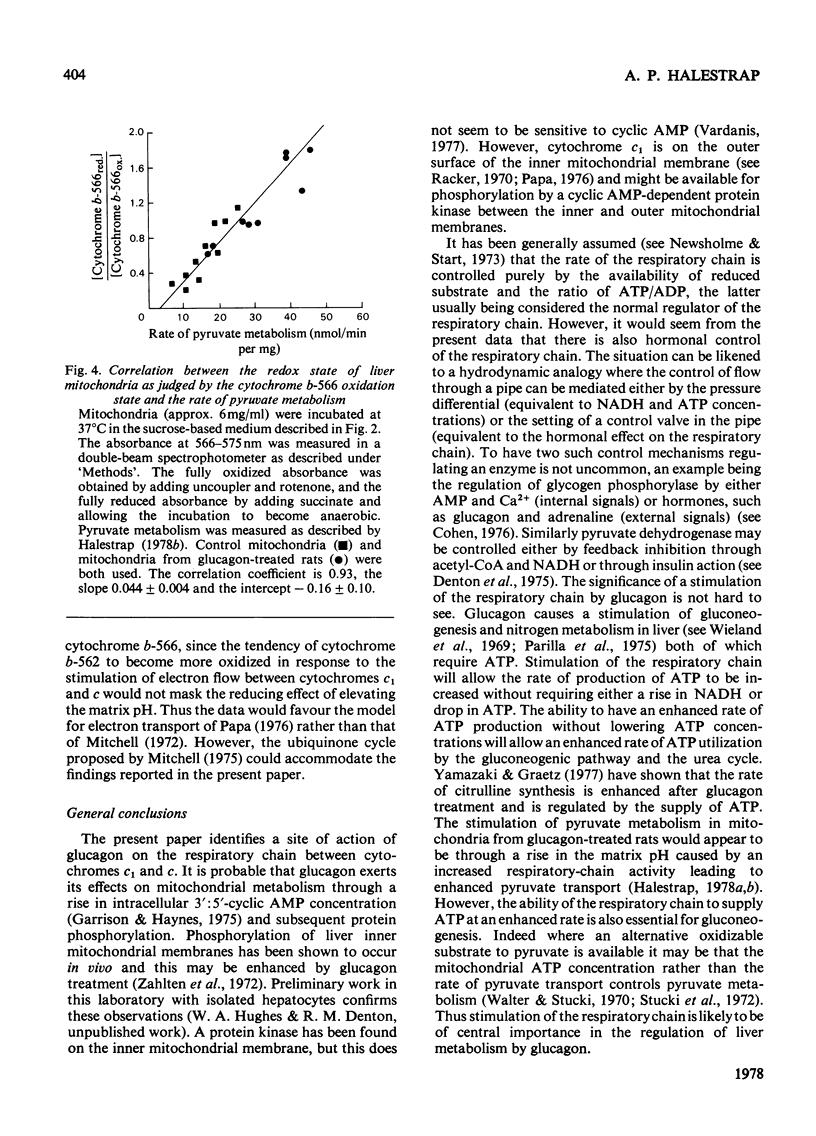
Mitochondria from glucagon-treated rats oxidize succinate, but not ascorbate plus tetramethylphenylenediamine, faster in the uncoupled state than do control mitochondria. The rate of O2 uptake in the presence of both substrates is equal to the sum of the rates of the O2 uptake in the presence of either substrate alone. It is concluded that the mitochondrial respiratory chain is limited at some point between cytochromes b and c and that this step is regulated by glucagon. Measurement of the cytochrome spectra under uncoupled conditions in the presence of succinate and rotenone demonstrates a crossover between cytochromes c and c1 when control mitochondria are compared with those from glucagon-treated rats, cytochrome c being more oxidized and cytochrome c1 more reduced in control mitochondria. Under conditions where pyruvate metabolism is studied the control mitochondria are generally more oxidized than those from glucagon-treated rats, the redox state of cytochrome b-566 correlating with the rate of pyruvate metabolism in sucrose medium. However, when the redox state of the mitochondria is taken into account, a crossover between cytochromes c and c1 is again apparent. The spectra of the b cytochromes are complex, but cytochrome b-562 appears to become more reduced relative to cytochrome b-566 in mitochondria from glucagon-treated rats than in control mitochondria. This can be explained by the existence of a more alkaline matrix in glucagon-treated rats, the redox potential for cytochrome b being pH-sensitive. It is concluded that glucagon stimulates electron flow between cytochromes c1 and c. The physiological significance of these findings is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam P. A., Haynes R. C., Jr Control of hepatic mitochondrial CO2 fixation by glucagon, epinephrine, and cortisol. J Biol Chem. 1969 Dec 10;244(23):6444–6450. [PubMed] [Google Scholar]
- Davis E. J., Blair P. V. Correlation of the steady-states of reduction of components of the respiratory chain with phosphorylation states of liver mitochondria. Biochem Biophys Res Commun. 1977 Aug 8;77(3):1017–1023. doi: 10.1016/s0006-291x(77)80079-x. [DOI] [PubMed] [Google Scholar]
- Denton R. M., Randle P. J., Bridges B. J., Cooper R. H., Kerbey A. L., Pask H. T., Severson D. L., Stansbie D., Whitehouse S. Regulation of mammalian pyruvate dehydrogenase. Mol Cell Biochem. 1975 Oct 31;9(1):27–53. doi: 10.1007/BF01731731. [DOI] [PubMed] [Google Scholar]
- Garrison J. C., Haynes R. C., Jr The hormonal control of gluconeogenesis by regulation of mitochondrial pyruvate carboxylation in isolated rat liver cells. J Biol Chem. 1975 Apr 25;250(8):2769–2777. [PubMed] [Google Scholar]
- Halestrap A. P. Pyruvate and ketone-body transport across the mitochondrial membrane. Exchange properties, pH-dependence and mechanism of the carrier. Biochem J. 1978 Jun 15;172(3):377–387. doi: 10.1042/bj1720377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap A. P. Stimulation of pyruvate transport in metabolizing mitochondria through changes in the transmembrane pH gradient induced by glucagon treatment of rats. Biochem J. 1978 Jun 15;172(3):389–398. doi: 10.1042/bj1720389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes R. C., Jr Mechanisms of hormonal control of gluconeogenesis. Metabolism. 1976 Nov;25(11 Suppl 1):1361–1363. doi: 10.1016/s0026-0495(76)80142-4. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Protonmotive redox mechanism of the cytochrome b-c1 complex in the respiratory chain: protonmotive ubiquinone cycle. FEBS Lett. 1975 Aug 1;56(1):1–6. doi: 10.1016/0014-5793(75)80098-6. [DOI] [PubMed] [Google Scholar]
- NORRIS K. H., BUTLER W. L. Techniques for obtaining absorption spectra on intact biological samples. Ire Trans Biomed Electron. 1961 Jul;8:153–157. doi: 10.1109/tbmel.1961.4322890. [DOI] [PubMed] [Google Scholar]
- Papa S. Proton translocation reactions in the respiratory chains. Biochim Biophys Acta. 1976 Apr 30;456(1):39–84. doi: 10.1016/0304-4173(76)90008-2. [DOI] [PubMed] [Google Scholar]
- Parrilla R., Jimenez I., Ayuso-Parrilla M. S. Glucagon and insulin control of gluconeogenesis in the perfused isolated rat liver. Effects on cellular metabolite distribution. Eur J Biochem. 1975 Aug 15;56(2):375–383. doi: 10.1111/j.1432-1033.1975.tb02243.x. [DOI] [PubMed] [Google Scholar]
- Racker E. The two faces of the inner mitochondrial membrane. Essays Biochem. 1970;6:1–22. [PubMed] [Google Scholar]
- Stucki J. W., Brawand F., Walter P. Regulation of pyruvate metabolim in rat-liver mitochondria by adenine nucleotides and fatty acids. Eur J Biochem. 1972 May;27(1):181–191. doi: 10.1111/j.1432-1033.1972.tb01824.x. [DOI] [PubMed] [Google Scholar]
- Titheradge M. A., Coore H. G. The mitochondrial pyruvate carrier, its exchange properties and its regulation by glucagon. FEBS Lett. 1976 Mar 15;63(1):45–50. doi: 10.1016/0014-5793(76)80191-3. [DOI] [PubMed] [Google Scholar]
- Verdanis A. Protein kinase activity at the inner membrane of mammalian mitochondria. J Biol Chem. 1977 Feb 10;252(3):807–813. [PubMed] [Google Scholar]
- Walter P., Stucki J. W. Regulation of pyruvate carboxylase in rat liver mitochondria by adenine nucleotides and short chain fatty acids. Eur J Biochem. 1970 Feb;12(3):508–519. doi: 10.1111/j.1432-1033.1970.tb00880.x. [DOI] [PubMed] [Google Scholar]
- Wilson D. F., Owen C. S., Holian A. Control of mitochondrial respiration: a quantitative evaluation of the roles of cytochrome c and oxygen. Arch Biochem Biophys. 1977 Aug;182(2):749–762. doi: 10.1016/0003-9861(77)90557-4. [DOI] [PubMed] [Google Scholar]
- Yamazaki R. K. Glucagon stimulation of mitochondrial respiration. J Biol Chem. 1975 Oct 10;250(19):7924–7930. [PubMed] [Google Scholar]
- Yamazaki R. K., Graetz G. S. Glucagon stimulation of citrulline formation in isolated hepatic mitochondria. Arch Biochem Biophys. 1977 Jan 15;178(1):19–25. doi: 10.1016/0003-9861(77)90166-7. [DOI] [PubMed] [Google Scholar]
- Yamazaki R. K., Sax R. D., Hauser M. A. Glucagon stimulation of mitochondrial ATPase and potassium ion transport. FEBS Lett. 1977 Mar 15;75(1):295–299. doi: 10.1016/0014-5793(77)80106-3. [DOI] [PubMed] [Google Scholar]
- Zahlten R. N., Hochberg A. A., Stratman F. W., Lardy H. A. Glucagon-stimulated phosphorylation of mitochondrial and lysosomal membranes of rat liver in vivo. Proc Natl Acad Sci U S A. 1972 Apr;69(4):800–804. doi: 10.1073/pnas.69.4.800. [DOI] [PMC free article] [PubMed] [Google Scholar]


