Abstract
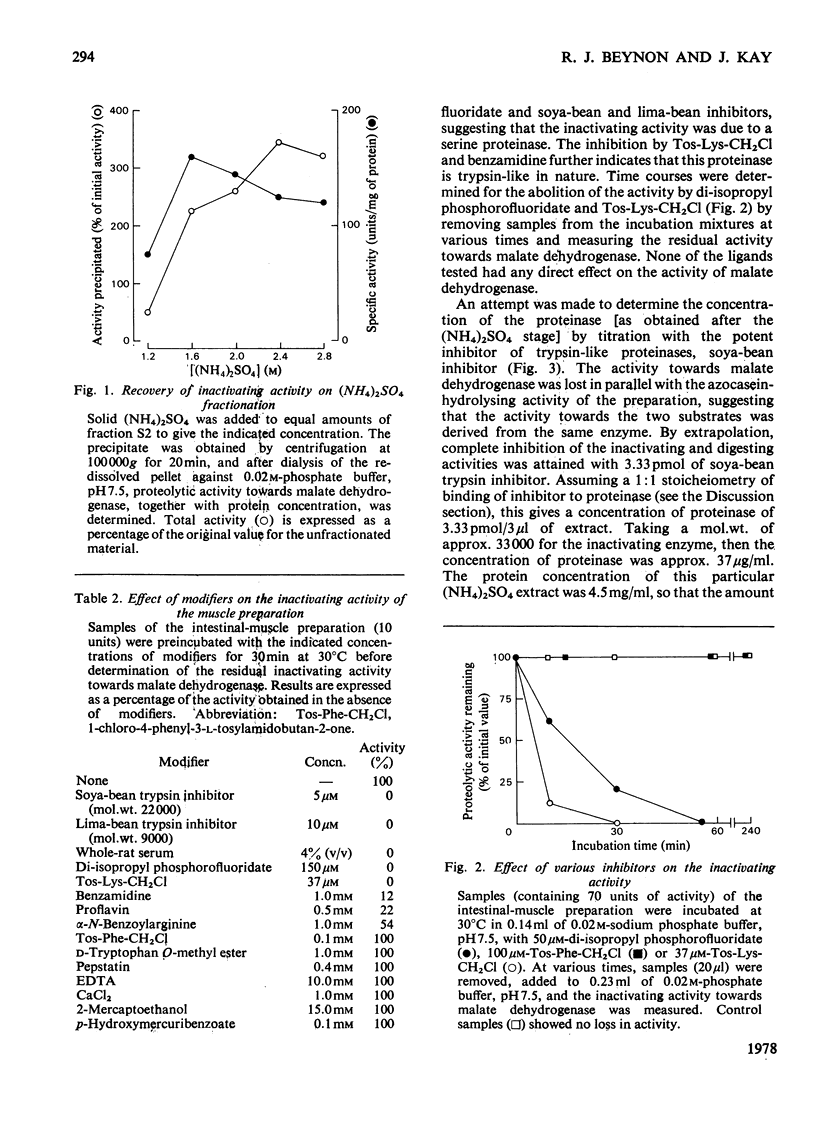
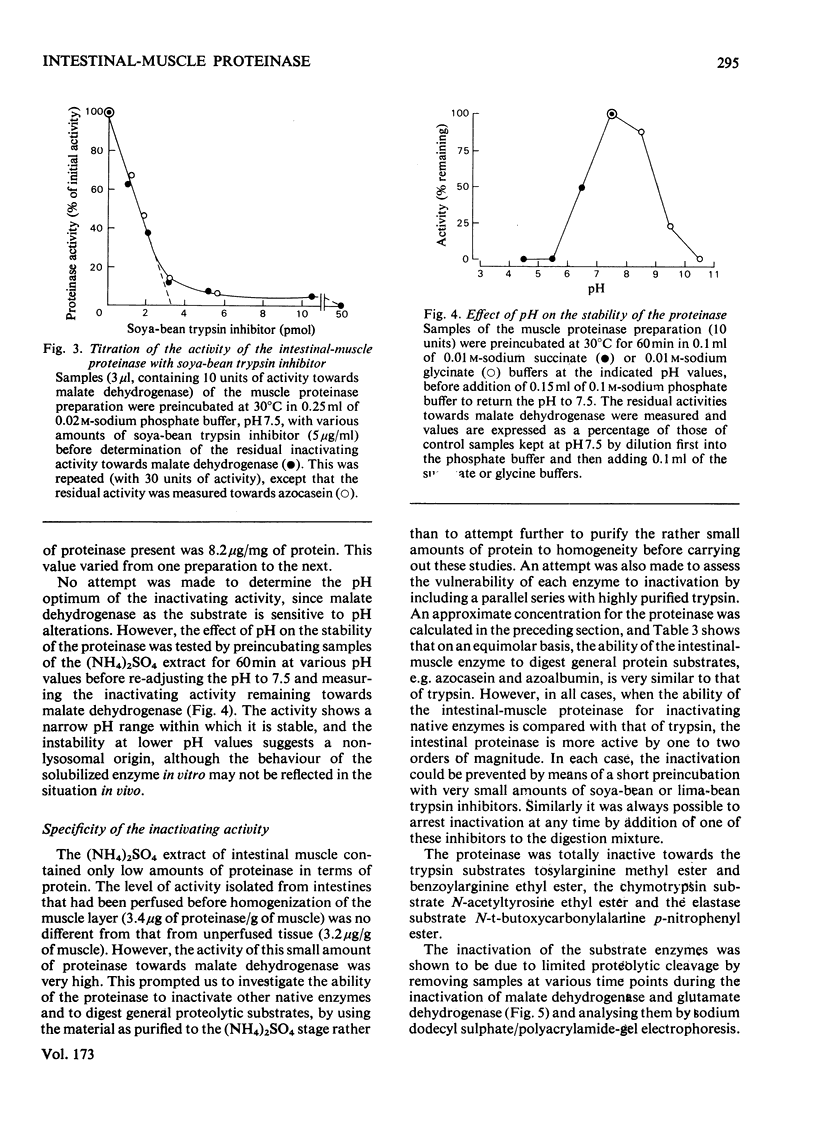
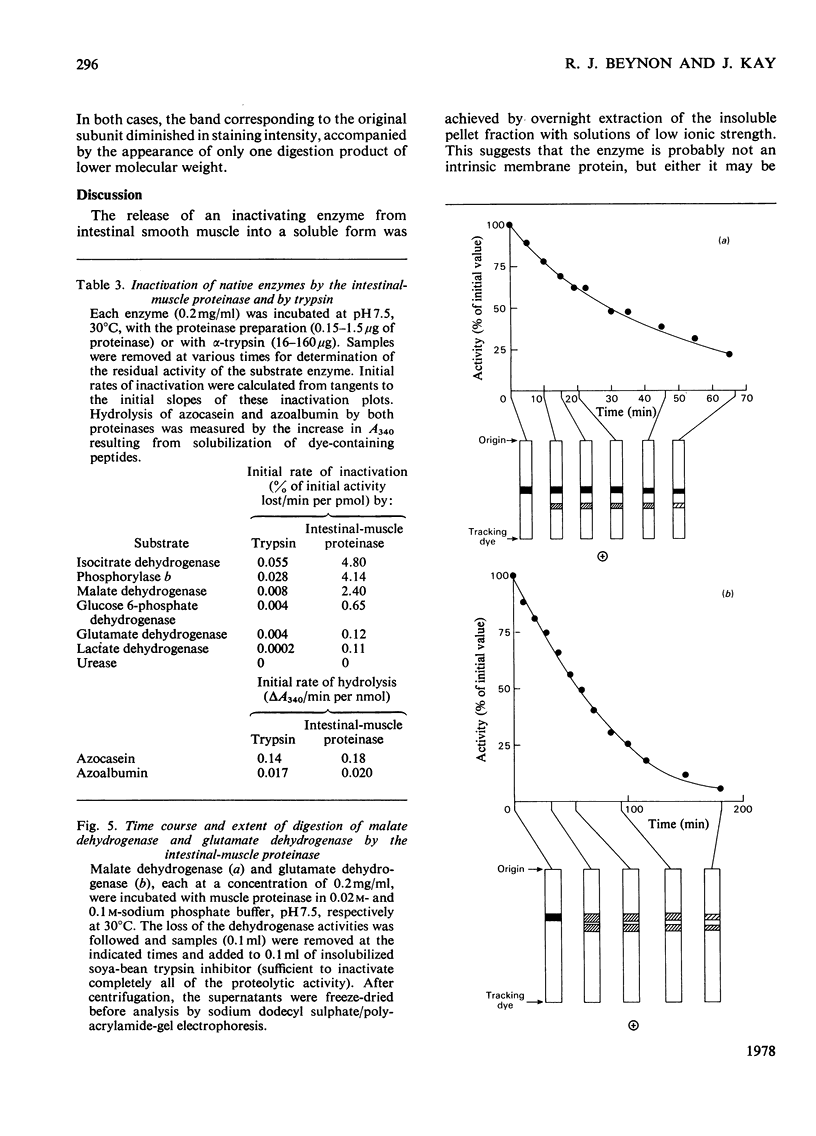
1. The solubilization and partial purification of a proteinase from the intestinal smooth muscle of rats fed on protein-free diets are described. 2. It has a mol.wt. of about 33000 and it is stable over a narrow pH range. 3. From its susceptibility to known modifers of proteolytic enzymes, it appears to be a serine proteinase of a trypsin-like nature. Active-site titration with soya-bean trypsin inhibitor shows that the concentration of proteinase was about 3 microgram/g wet wt. of intestinal smooth muscle. However, the muscle proteinase demonstrates a marked ability for inactivating enzymes in their native conformation at neutral pH. It is about 100 times more efficient than pancreatic trypsin when the inactivating activities are compared on an approximately equimolar basis. 4. Inactivation of the substrate enzymes is accompanied by limited proteolysis, as demonstrated by sodium dodecyl sulphate/polyacrylamide-gel electrophoresis. 5. An endogenous inhibitor was separated from the proteinase by fractionation with (NH4)2SO4. 6. Contamination of the muscle tissue by lumen, mucosal or blood proteinases and inhibitors is shown to be unlikely. 7. A role for the neutral trypsin-like proteinase in initiating the degradation of intracellular enzymes is considered.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dayton W. R., Goll D. E., Zeece M. G., Robson R. M., Reville W. J. A Ca2+-activated protease possibly involved in myofibrillar protein turnover. Purification from porcine muscle. Biochemistry. 1976 May 18;15(10):2150–2158. doi: 10.1021/bi00655a019. [DOI] [PubMed] [Google Scholar]
- Duckworth W. C., Heinemann M., Kitabchi A. E. Proteolytic degradation of insulin and glucagon. Biochim Biophys Acta. 1975 Feb 19;377(2):421–430. doi: 10.1016/0005-2744(75)90322-8. [DOI] [PubMed] [Google Scholar]
- HUMMEL B. C. A modified spectrophotometric determination of chymotrypsin, trypsin, and thrombin. Can J Biochem Physiol. 1959 Dec;37:1393–1399. [PubMed] [Google Scholar]
- Jusic M., Seifert S., Weiss E., Haas R., Heinrich P. C. Isolation and characterization of a membrane-bound proteinase from rat liver. Arch Biochem Biophys. 1976 Dec;177(2):355–363. doi: 10.1016/0003-9861(76)90449-5. [DOI] [PubMed] [Google Scholar]
- Katunuma N., Kominami E., Kobayashi K., Banno Y., Suzuki K. Studies on new intracellular proteases in various organs of rat. 1. Purification and comparison of their properties. Eur J Biochem. 1975 Mar 3;52(1):37–50. doi: 10.1111/j.1432-1033.1975.tb03970.x. [DOI] [PubMed] [Google Scholar]
- Katz J., Troll W., Levy M., Filkins K., Russo J., Levitz M. Estrogen-dependent trypsin-like activity in the rat uterus. Localization of activity in the 12,000g pellet and nucleus. Arch Biochem Biophys. 1976 Mar;173(1):347–354. doi: 10.1016/0003-9861(76)90269-1. [DOI] [PubMed] [Google Scholar]
- Kawiak J., Vensel W. H., Komender J., Barnard E. A. Non-pancreatic proteases of the chymotrypsin family. I. A chymotrypsin-like protease from rat mast cells. Biochim Biophys Acta. 1971 Apr 14;235(1):172–187. doi: 10.1016/0005-2744(71)90045-3. [DOI] [PubMed] [Google Scholar]
- Kay J., Kassell B. The autoactivation of trypsinogen. J Biol Chem. 1971 Nov;246(21):6661–6665. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Schroeder D. D., Shaw E. Chromatography of trypsin and its derivatives. Characterization of a new active form of bovine trypsin. J Biol Chem. 1968 Jun 10;243(11):2943–2949. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Woessner J. F., Jr A latent form of collagenase in the involuting rat uterus and its activation by a serine proteinase. Biochem J. 1977 Mar 1;161(3):535–542. doi: 10.1042/bj1610535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright H. T. Secondary and conformational specificities of trypsin and chymotrypsin. Eur J Biochem. 1977 Mar 1;73(2):567–578. doi: 10.1111/j.1432-1033.1977.tb11352.x. [DOI] [PubMed] [Google Scholar]


