Abstract
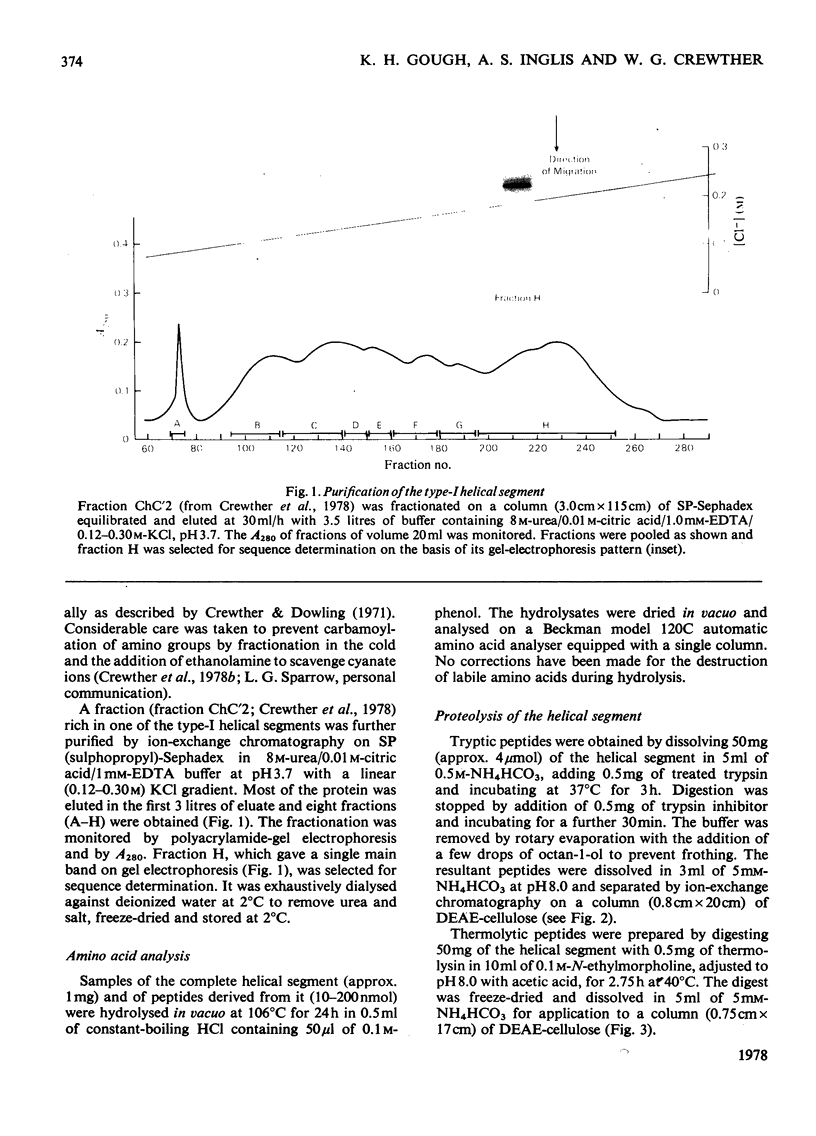
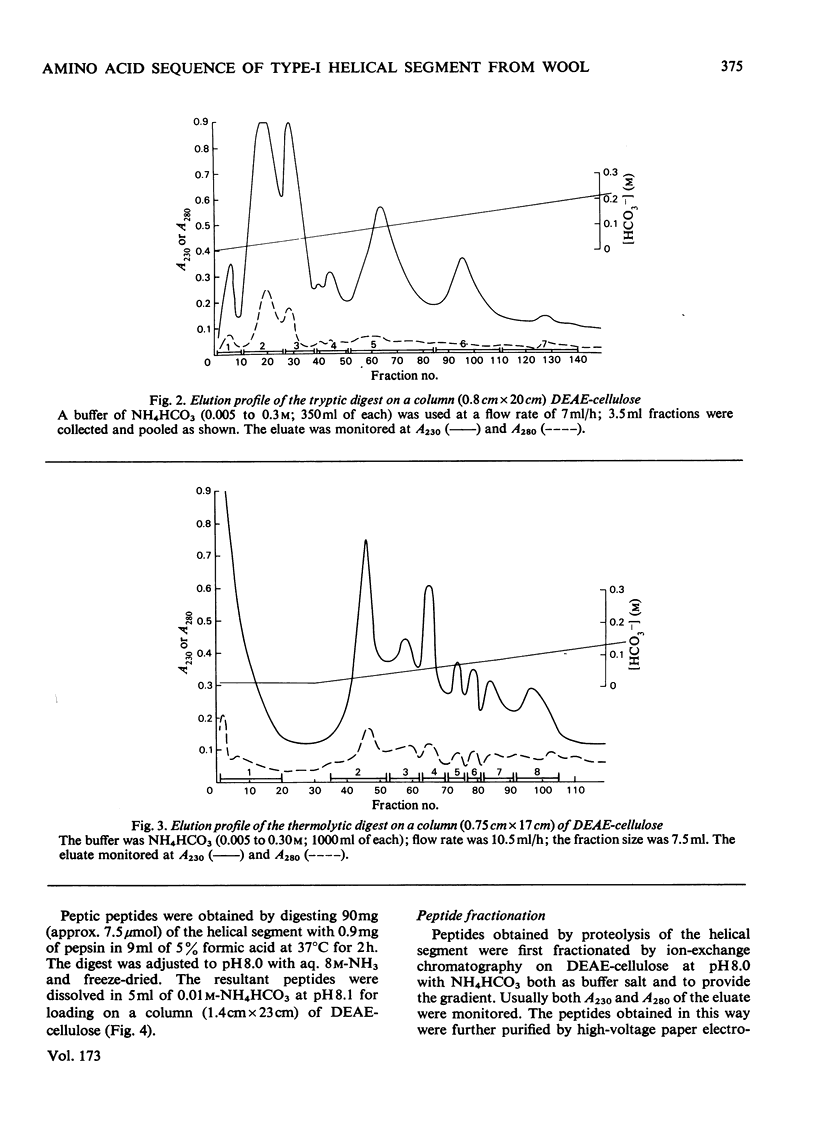
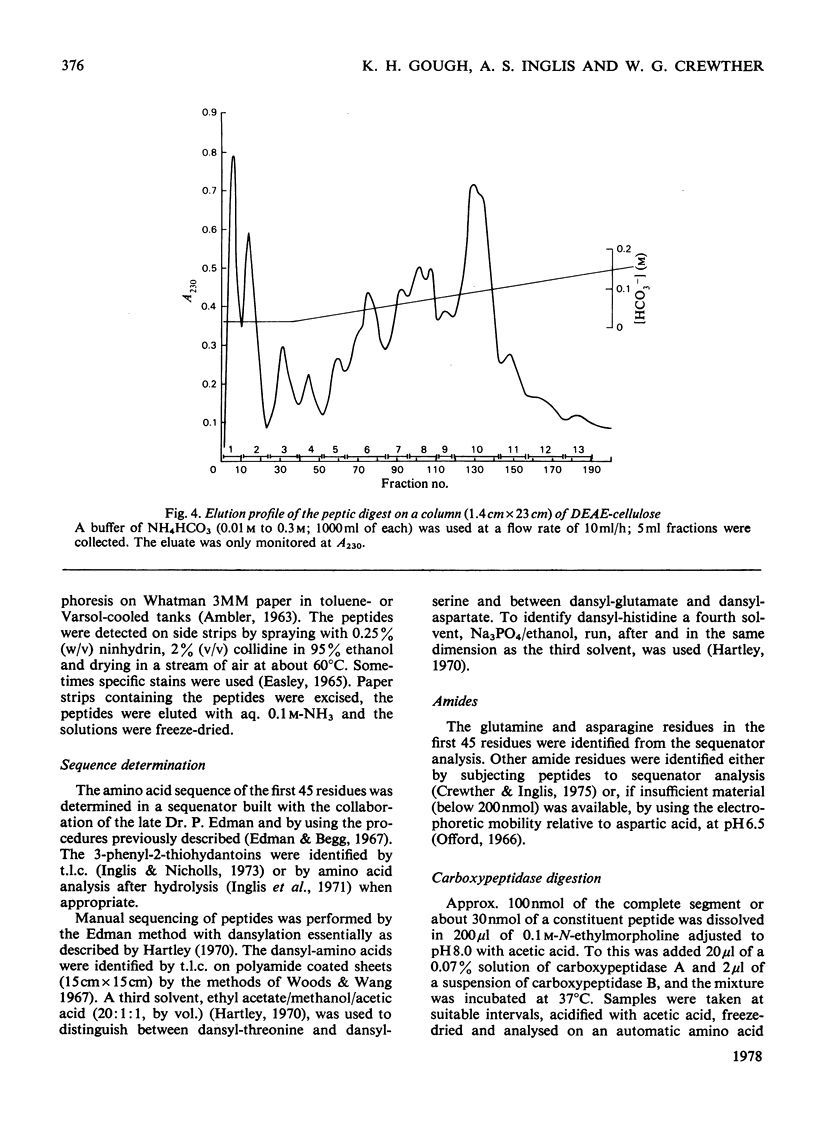
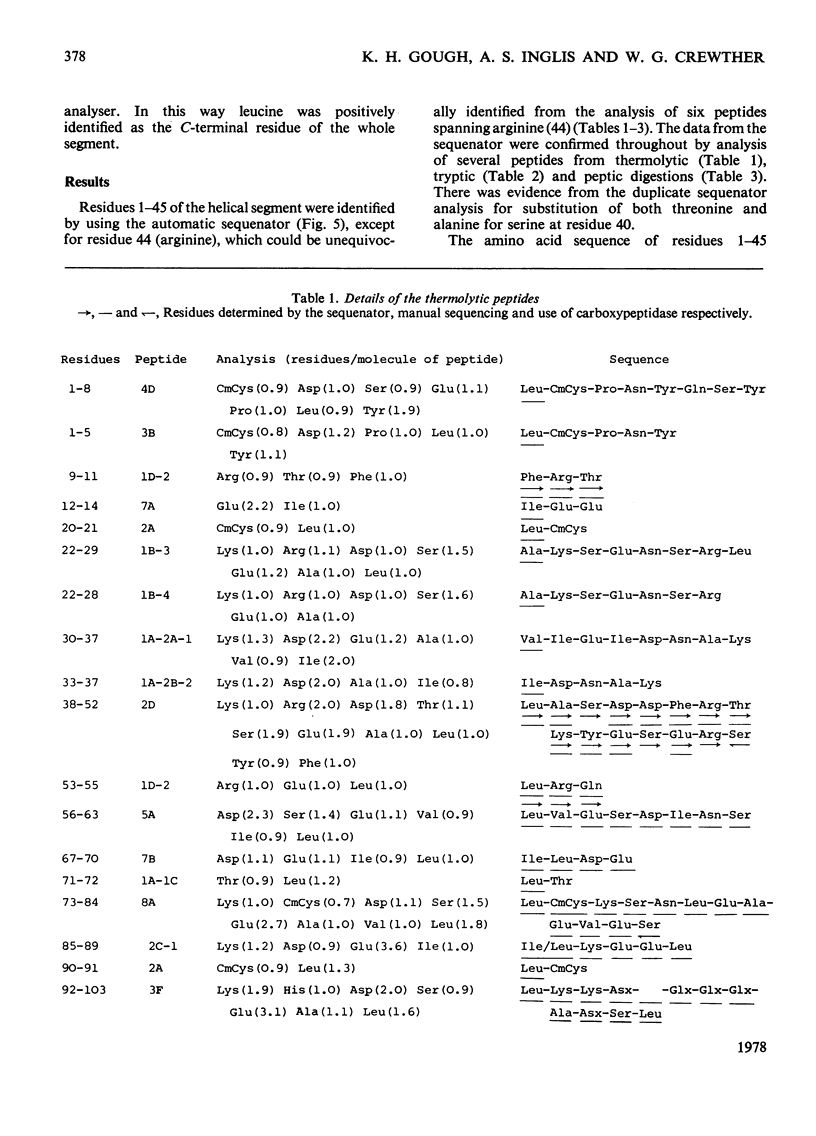
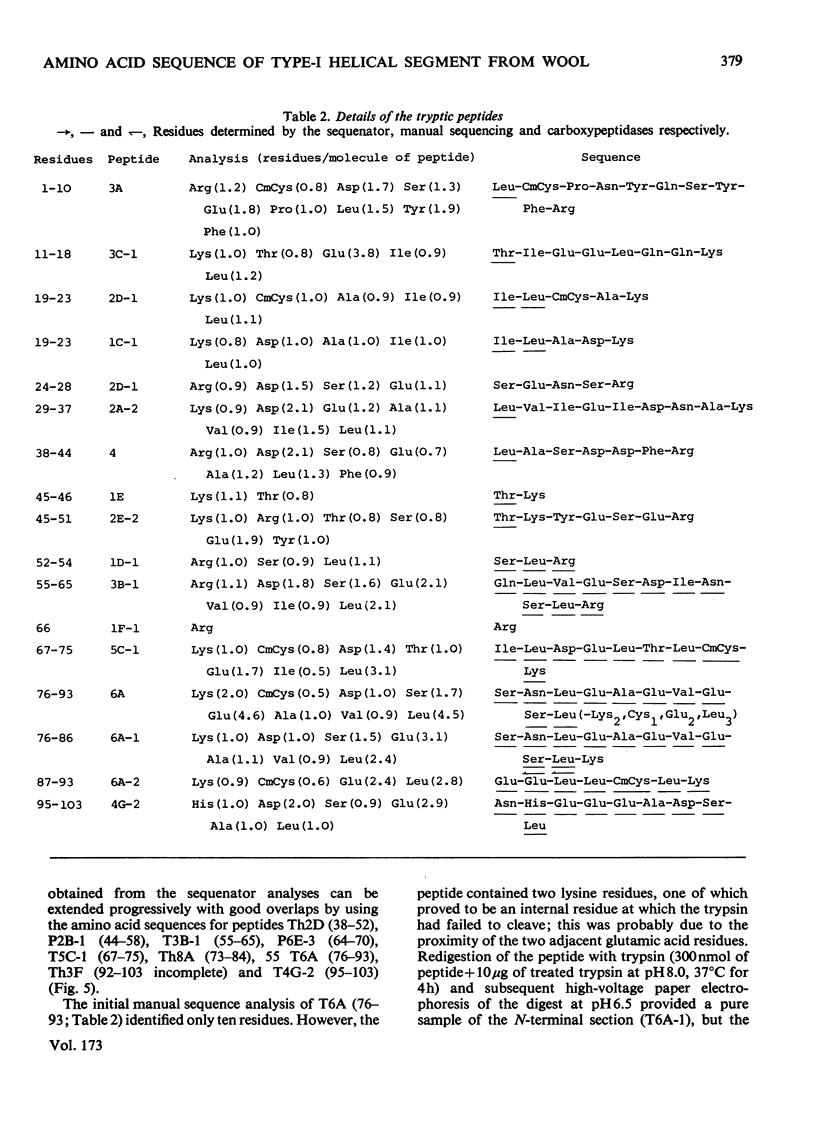
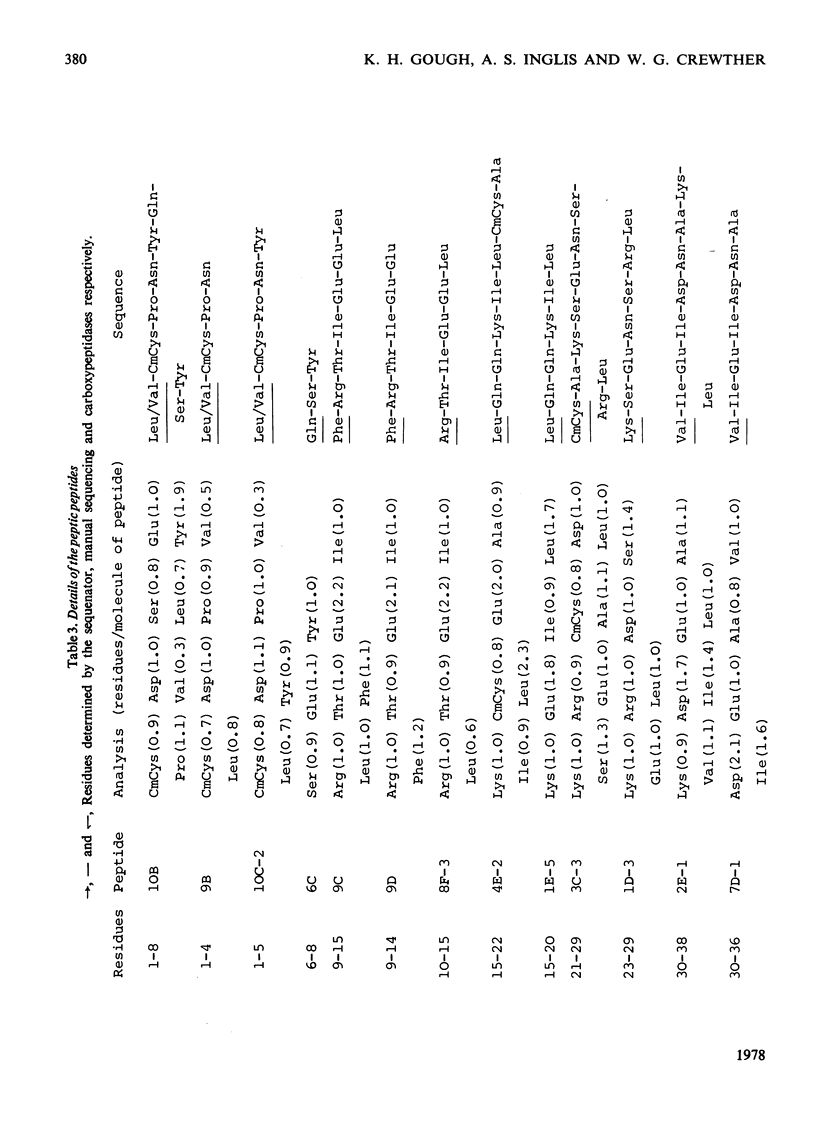
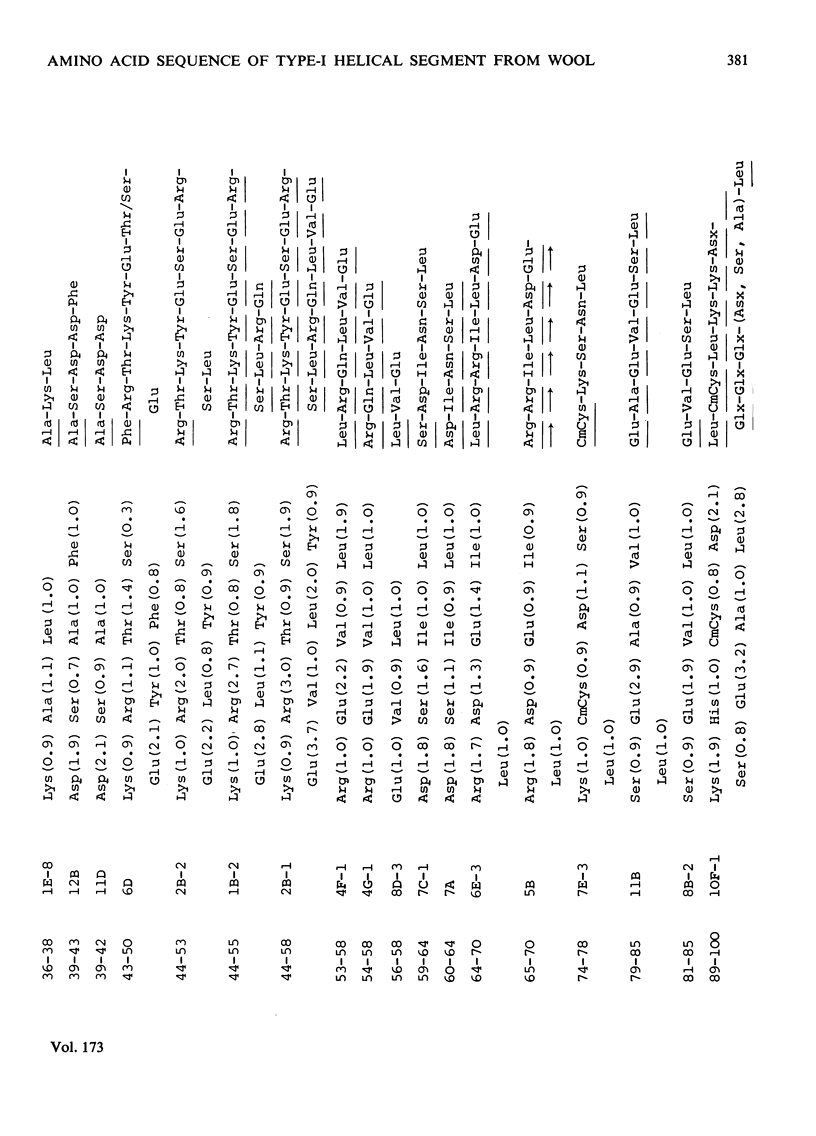
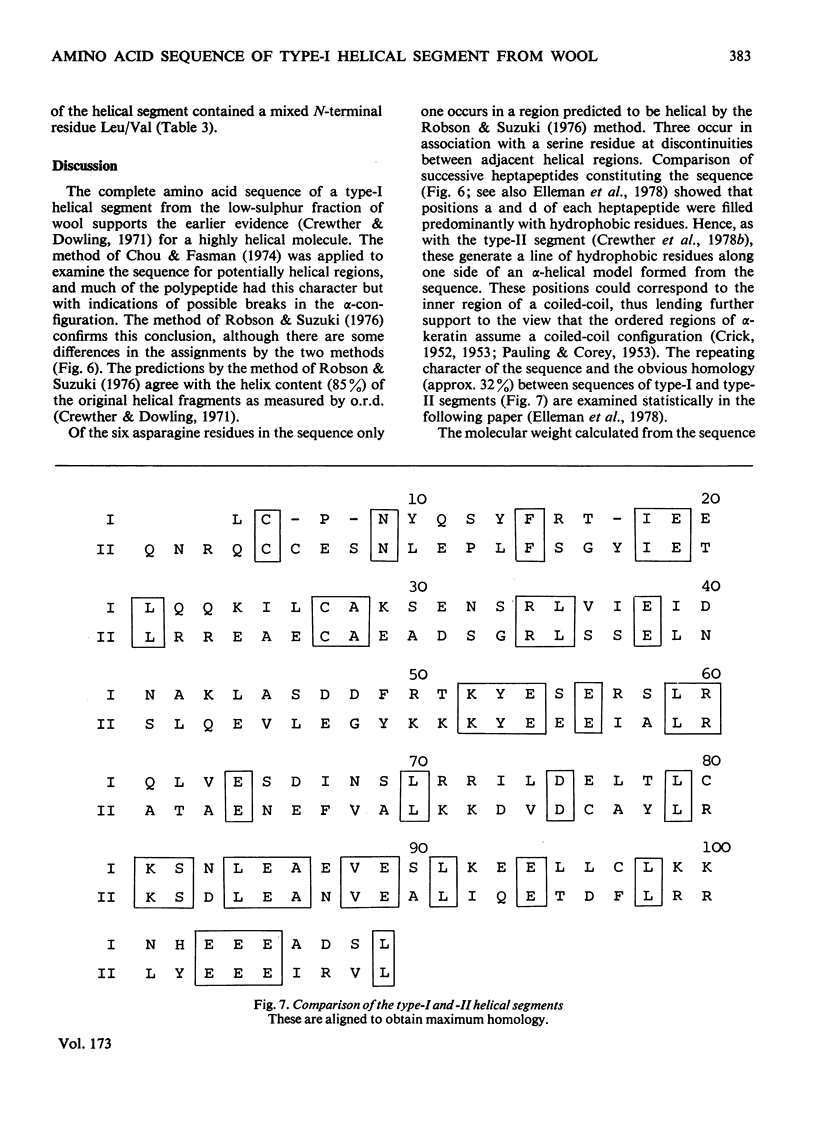
The amino acid sequence of a type-I helical segment from the low-sulphur protein (S-carboxymethylkerateine-A) of wool was determined by combining automatic and manual-sequencing data. Whereas in the type-II helical segment most of the cationic groups occur in pairs, 11 of the 22 anionic residues in the sequence of the type-I segment were situated next to a second anionic residue. This suggests possible interactions between type-I and type-II helical segments in alpha-keratin. As observed with the sequence of a type-II helical segment a model constructed on 3.6 residues per turn of helix shows a line of hydrophobic residues along the helix, thereby supporting the physicochemical evidence that the molecule is predominantly helical and forms part of a coiled-coil structure. Examination of the sequence data by predictive methods indicates the possibilty of extensive sections of alpha-helix interspersed with discontinuities. The molecule contains a number of regions with peptide sequences identical with those found by other workers after enzymic digestion of fractions from oxidized wool.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBLER R. P. THE AMINO ACID SEQUENCE OF PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:349–378. doi: 10.1042/bj0890349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRICK F. H. C. Is alpha-keratin a coiled coil? Nature. 1952 Nov 22;170(4334):882–883. doi: 10.1038/170882b0. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Corfield M. C., Fletcher J. C. Amino acid sequences of peptides from a chymotryptic digest of a urea-soluble protein fraction (U.S.3) from oxidized wool. Biochem J. 1969 Nov;115(2):323–334. doi: 10.1042/bj1150323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corfield M. C., Fletcher J. C., Robson A. Amino acid sequences of peptides from a tryptic digest of a urea-soluble protein fraction (U.S.3) from oxidized wool. Biochem J. 1967 Mar;102(3):801–814. doi: 10.1042/bj1020801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crewther W. G., Harrap B. S. The preparation and properties of a helix-rich fraction obtained by partial proteolysis of low sulfur S-carboxymethylkerateine from wool. J Biol Chem. 1967 Oct 10;242(19):4310–4319. [PubMed] [Google Scholar]
- Crewther W. G., Inglis A. S. Automatic procedures for determining amino acid sequences of peptides. Anal Biochem. 1975 Oct;68(2):572–585. doi: 10.1016/0003-2697(75)90653-3. [DOI] [PubMed] [Google Scholar]
- Crewther W. G., Inglis A. S., McKern N. M. Amino acid sequences of alpha-helical segments from S-carboxymethylkerateine-A. Complete sequence of a type-II segment. Biochem J. 1978 Aug 1;173(2):365–371. doi: 10.1042/bj1730365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easley C. W. Combinations of specific color reactions useful in the peptide mapping technique. Biochim Biophys Acta. 1965 Sep 13;107(2):386–388. doi: 10.1016/0304-4165(65)90147-9. [DOI] [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Elleman T. C., Crewther W. G., Van Der Touw J. Amino acid sequences of alpha-helical segments from S-carboxymethylkerateine-A. Statistical analysis. Biochem J. 1978 Aug 1;173(2):387–391. doi: 10.1042/bj1730387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H., HARRIS J. I., LEVY A. L. Recent developments in techniques for terminal and sequence studies in peptides and proteins. Methods Biochem Anal. 1955;2:359–425. doi: 10.1002/9780470110188.ch12. [DOI] [PubMed] [Google Scholar]
- Hartley B. S. Strategy and tactics in protein chemistry. Biochem J. 1970 Oct;119(5):805–822. doi: 10.1042/bj1190805f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg D. M., Dowling L. M., Crewther W. G. Amino acid sequences of alpha-helical segments from S-carboxymethylkerateine-A. Tryptic and chymotryptic peptides from a type-II segment. Biochem J. 1978 Aug 1;173(2):353–363. doi: 10.1042/bj1730353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis A. S., Nicholls P. W. Identification of phenylthiohydantoins of amino acids by thin-layer chromatography. J Chromatogr. 1973 May 16;79:344–346. doi: 10.1016/s0021-9673(01)85310-3. [DOI] [PubMed] [Google Scholar]
- Inglis A. S., Nicholls P. W., Roxburgh C. M. Hydrolysis of the peptide bond and amino acid modification with hydriodic acid. Aust J Biol Sci. 1971 Dec;24(6):1235–1240. doi: 10.1071/bi9711235. [DOI] [PubMed] [Google Scholar]
- Moffitt W., Yang J. T. THE OPTICAL ROTATORY DISPERSION OF SIMPLE POLYPEPTIDES. I. Proc Natl Acad Sci U S A. 1956 Sep;42(9):596–603. doi: 10.1073/pnas.42.9.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- PAULING L., COREY R. B. Compound helical configurations of polypeptide chains: structure of proteins of the alpha-keratin type. Nature. 1953 Jan 10;171(4341):59–61. doi: 10.1038/171059a0. [DOI] [PubMed] [Google Scholar]
- Parry D. A., Crewther W. G., Fraser R. D., MacRae T. P. Structure of alpha-keratin: structural implication of the amino acid sequences of the type I and type II chain segments. J Mol Biol. 1977 Jun 25;113(2):449–454. doi: 10.1016/0022-2836(77)90153-x. [DOI] [PubMed] [Google Scholar]
- Robson B., Suzuki E. Conformational properties of amino acid residues in globular proteins. J Mol Biol. 1976 Nov 5;107(3):327–356. doi: 10.1016/s0022-2836(76)80008-3. [DOI] [PubMed] [Google Scholar]
- Woods K. R., Wang K. T. Separation of dansyl-amino acids by polyamide layer chromatography. Biochim Biophys Acta. 1967 Feb 21;133(2):369–370. doi: 10.1016/0005-2795(67)90078-5. [DOI] [PubMed] [Google Scholar]



