Abstract
The binding of beta-methyl N-acetylglucosaminide (betaMeGlcNAc) to egg-white lysozyme of hen in the tetragonal crystal form was studied by X-ray diffraction techniques to a resolution of 0.25 nm. The binding of the beta-methyl glycoside is almost identical with the binding of beta-N-acetylglucosamine (betaGlcNAc). Real-space refinement of the lysozyme-alpha/beta GlcNAc and lysozyme-betaMeGlcNAc complexes allowed preliminary analysis of the conformational changes observed on binding monosaccharide inhibitors, specially in the region involving tryptophan-62 and residues 70--76. Tetagonal lysozyme crystals, grown in the absence of acetate ions, were examined by X-ray diffraction to 0.25nm resolution. The resulting difference Fourier synthesis shows no firm evidence for bound acetate ions and indicates only minor conformational changes in the side-chain positions of aspartic acid-101 and asparagine-103. The close similarity of the lysozyme structures in the presence and absence of acetate is contrary to expectations from previous n.m.r. studies.
Full text
PDF


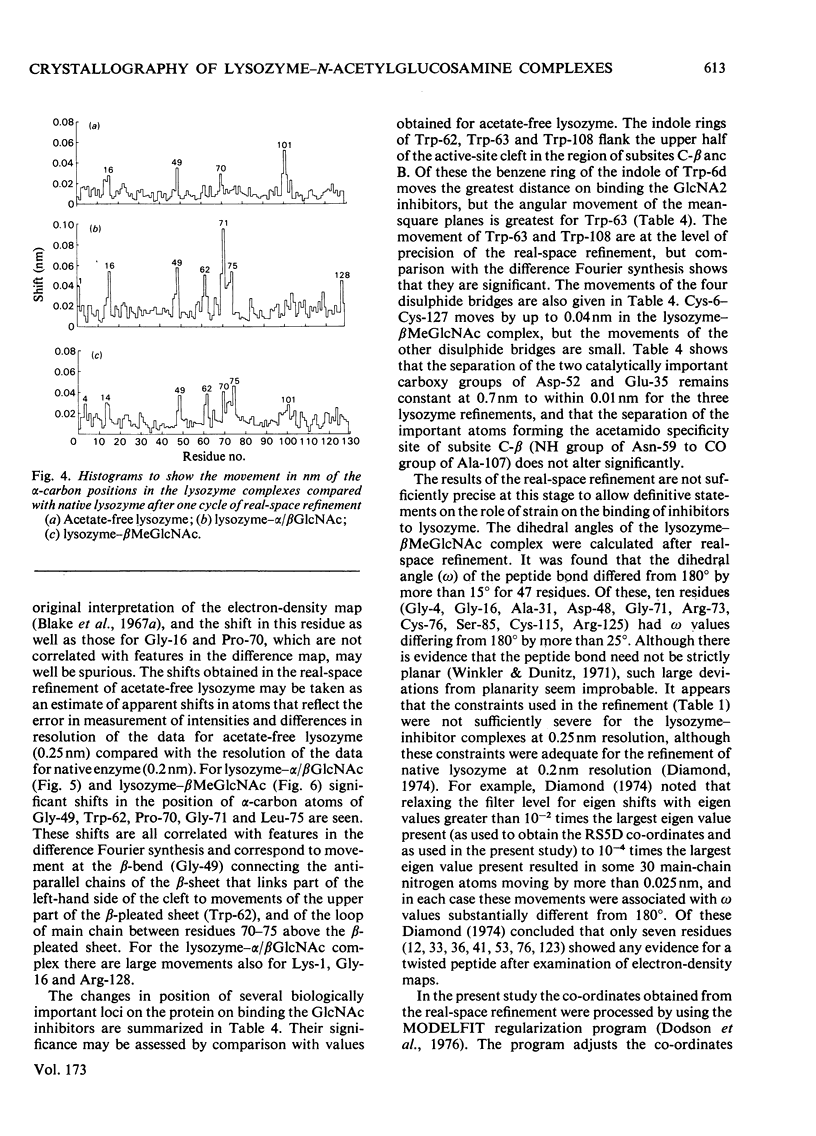
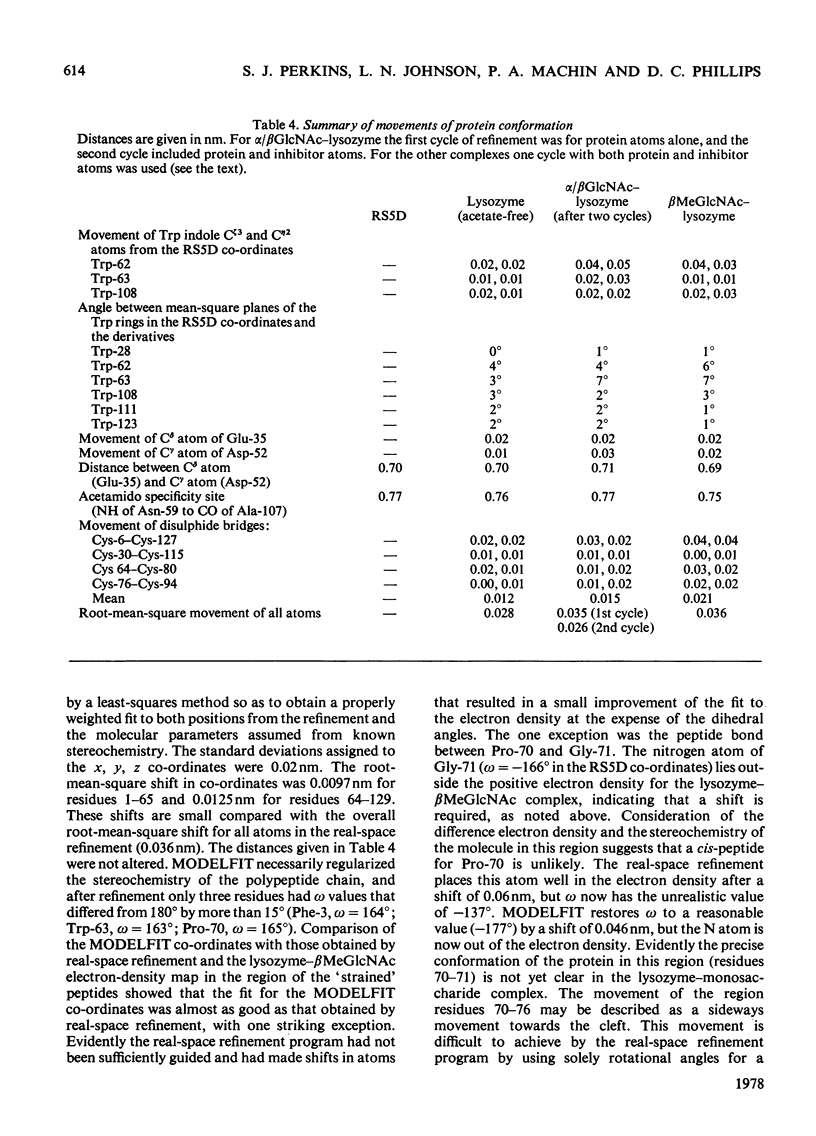


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake C. C., Johnson L. N., Mair G. A., North A. C., Phillips D. C., Sarma V. R. Crystallographic studies of the activity of hen egg-white lysozyme. Proc R Soc Lond B Biol Sci. 1967 Apr 18;167(1009):378–388. doi: 10.1098/rspb.1967.0035. [DOI] [PubMed] [Google Scholar]
- Blake C. C., Koenig D. F., Mair G. A., North A. C., Phillips D. C., Sarma V. R. Structure of hen egg-white lysozyme. A three-dimensional Fourier synthesis at 2 Angstrom resolution. Nature. 1965 May 22;206(4986):757–761. doi: 10.1038/206757a0. [DOI] [PubMed] [Google Scholar]
- Blake C. C., Mair G. A., North A. C., Phillips D. C., Sarma V. R. On the conformation of the hen egg-white lysozyme molecule. Proc R Soc Lond B Biol Sci. 1967 Apr 18;167(1009):365–377. doi: 10.1098/rspb.1967.0034. [DOI] [PubMed] [Google Scholar]
- Diamond R. Real-space refinement of the structure of hen egg-white lysozyme. J Mol Biol. 1974 Jan 25;82(3):371–391. doi: 10.1016/0022-2836(74)90598-1. [DOI] [PubMed] [Google Scholar]
- Johnson L. N. The crystal structure of N-acetyl-alpha-D-glucosamine. Acta Crystallogr. 1966 Dec 10;21(6):885–891. doi: 10.1107/s0365110x66004146. [DOI] [PubMed] [Google Scholar]
- Jones R., Dwek R. A. The mechanism of water-proton relaxation in enzyme paramagnetic-ion complexes. 1. The Gd(3)-lysozyme complex. Eur J Biochem. 1974 Sep 1;47(2):271–283. doi: 10.1111/j.1432-1033.1974.tb03691.x. [DOI] [PubMed] [Google Scholar]
- Kurachi K., Sieker L. C., Jensen L. H. Structures of triclinic mono- and di-N-acetylglucosamine: lysozyme complexes--a crystallographic study. J Mol Biol. 1976 Feb 15;101(1):11–24. doi: 10.1016/0022-2836(76)90063-2. [DOI] [PubMed] [Google Scholar]
- Levitt M. Energy refinement of hen egg-white lysozyme. J Mol Biol. 1974 Jan 25;82(3):393–420. doi: 10.1016/0022-2836(74)90599-3. [DOI] [PubMed] [Google Scholar]
- Perkins S. J., Johnson L. N., Phillips D. C., Dwek R. A. Conformational changes, dynamics and assignments in 1H NMR studies of proteins using ring current calculations. Hen egg white lysozyme. FEBS Lett. 1977 Oct 1;82(1):17–22. doi: 10.1016/0014-5793(77)80876-4. [DOI] [PubMed] [Google Scholar]
- Shindo H., Cohen J. S. Observation of individual carboxyl groups in hen egg-white lysozyme by use of high field 13C-nuclear magnetic resonance. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1979–1983. doi: 10.1073/pnas.73.6.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrake A., Rupley J. A. Environment and exposure to solvent of protein atoms. Lysozyme and insulin. J Mol Biol. 1973 Sep 15;79(2):351–371. doi: 10.1016/0022-2836(73)90011-9. [DOI] [PubMed] [Google Scholar]
- Snape K. W., Tjian R., Blake C. C., Koshland D. E. Crystallographic study of the interaction of urea with lysozyme. Nature. 1974 Jul 26;250(464):295–298. doi: 10.1038/250295a0. [DOI] [PubMed] [Google Scholar]
- Sykes B. D. A transient nuclear magnetic resonance study of the kinetics of methyl N-acetyl-D-glucosaminide inhibition of lysozyme. Biochemistry. 1969 Mar;8(3):1110–1116. doi: 10.1021/bi00831a043. [DOI] [PubMed] [Google Scholar]
- Winkler F. K., Dunitz J. D. The non-planar amide group. J Mol Biol. 1971 Jul 14;59(1):169–182. doi: 10.1016/0022-2836(71)90419-0. [DOI] [PubMed] [Google Scholar]