Abstract
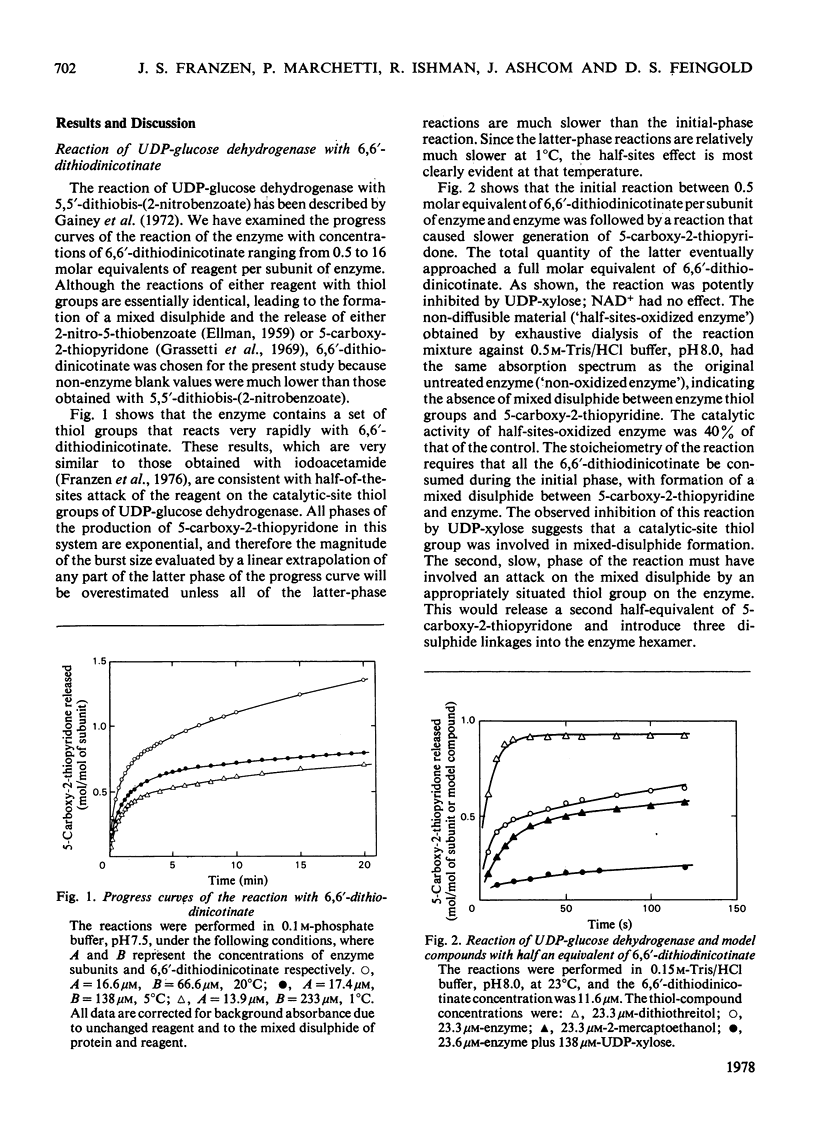
6,6-Dithiodinicotinate shows half-of-the-sites reactivity towards the six catalytic-site thiol groups of bovine liver UDP-glucose dehydrogenase. The reagent introduces three intrasubunit disulphide linkages between catalytic-site thiol groups and non-catalytic-site thiol groups and abrogates 60% of the catalytic activity of the hexameric enzyme; excess 2-mercaptoethanol rapidly restores full catalytic activity. These results show the half-of-the-sites behaviour of the enzyme with the reagent and the presence of a non-catalytic-site thiol group capable of forming a disulphide linkage with a catalytic-site thiol group on the same subunit without irreversible denaturation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen A., Marchetti P., Weingarten M., Franzen J., Feingold D. S. Binding studies with bovine liver UPD-D-glucose dehydrogenase. Carbohydr Res. 1974 Oct;37(1):155–165. doi: 10.1016/s0008-6215(00)87071-8. [DOI] [PubMed] [Google Scholar]
- Collier H. B. Letter: A note on the molar absorptivity of reduced Ellman's reagent, 3-carboxylato-4-nitrothiophenolate. Anal Biochem. 1973 Nov;56(1):310–311. doi: 10.1016/0003-2697(73)90196-6. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Franzen J. S., Ishman R., Feingold D. S. Half-of-the-sites reactivity of bovine liver uridine diphosphoglucose dehydrogenase toward iodoacetate and iodoacetamide. Biochemistry. 1976 Dec 14;15(25):5665–5671. doi: 10.1021/bi00670a036. [DOI] [PubMed] [Google Scholar]
- Franzen J. S., Kuo I., Eichler A. J., Feingold D. S. UDP-glucose dehydrogenase: substrate binding stoichiometry and affinity. Biochem Biophys Res Commun. 1973 Jan 23;50(2):517–523. doi: 10.1016/0006-291x(73)90870-x. [DOI] [PubMed] [Google Scholar]
- Gainey P. A., Pestell T. C., Phelps C. F. A study of the subunit structure and the thiol reactivity of bovine liver uridine diphosphate glucose dehydrogenase. Biochem J. 1972 Oct;129(4):821–830. doi: 10.1042/bj1290821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainey P. A., Phelps C. F. The binding of oxidized and reduced nicotinamide--adenine dinucleotides to bovine liver uridine diphosphate glucose dehydrogenase. Biochem J. 1974 Sep;141(3):667–673. doi: 10.1042/bj1410667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassetti D. R., Murray J. F., Jr, Ruan H. T. The interaction of 6,6'-dithiodinicotinic acid with thiols and with ehrlich ascites tumor cells. Biochem Pharmacol. 1969 Mar;18(3):603–611. doi: 10.1016/0006-2952(69)90085-9. [DOI] [PubMed] [Google Scholar]
- HARRIS I., MERIWETHER B. P., PARK J. H. Chemical nature of the catalytic sites in glyceraldehyde-3-phosphate dehydrogenase. Nature. 1963 Apr 13;198:154–157. doi: 10.1038/198154a0. [DOI] [PubMed] [Google Scholar]
- Ordman A. B., Kirkwood S. Mechanism of action of uridine diphoglucose dehydrogenase. Evidence for an essential lysine residue at the active site. J Biol Chem. 1977 Feb 25;252(4):1320–1326. [PubMed] [Google Scholar]
- Parker D. J., Allison W. S. The mechanism of inactivation of glyceraldehyde 3-phosphate dehydrogenase by tetrathionate, o-iodosobenzoate, and iodine monochloride. J Biol Chem. 1969 Jan 10;244(1):180–189. [PubMed] [Google Scholar]
- Ridley W. P., Houchins J. P., Kirkwood S. Mechanism of action of uridine diphosphoglucose dehydrogenase. Evidence for a second reversible dehydrogenation step involving an essential thiol group. J Biol Chem. 1975 Nov 25;250(22):8761–8767. [PubMed] [Google Scholar]
- Wassarman P. M., Major J. P. The reactivity of the sulfhydryl groups of lobster muscle glyceraldehyde 3-phosphate dehydrogenase. Biochemistry. 1969 Mar;8(3):1076–1082. doi: 10.1021/bi00831a039. [DOI] [PubMed] [Google Scholar]
- Zalitis J., Feingold D. S. Purification and properties of UDPG dehydrogenase from beef liver. Arch Biochem Biophys. 1969 Jul;132(2):457–465. doi: 10.1016/0003-9861(69)90389-0. [DOI] [PubMed] [Google Scholar]


