Abstract
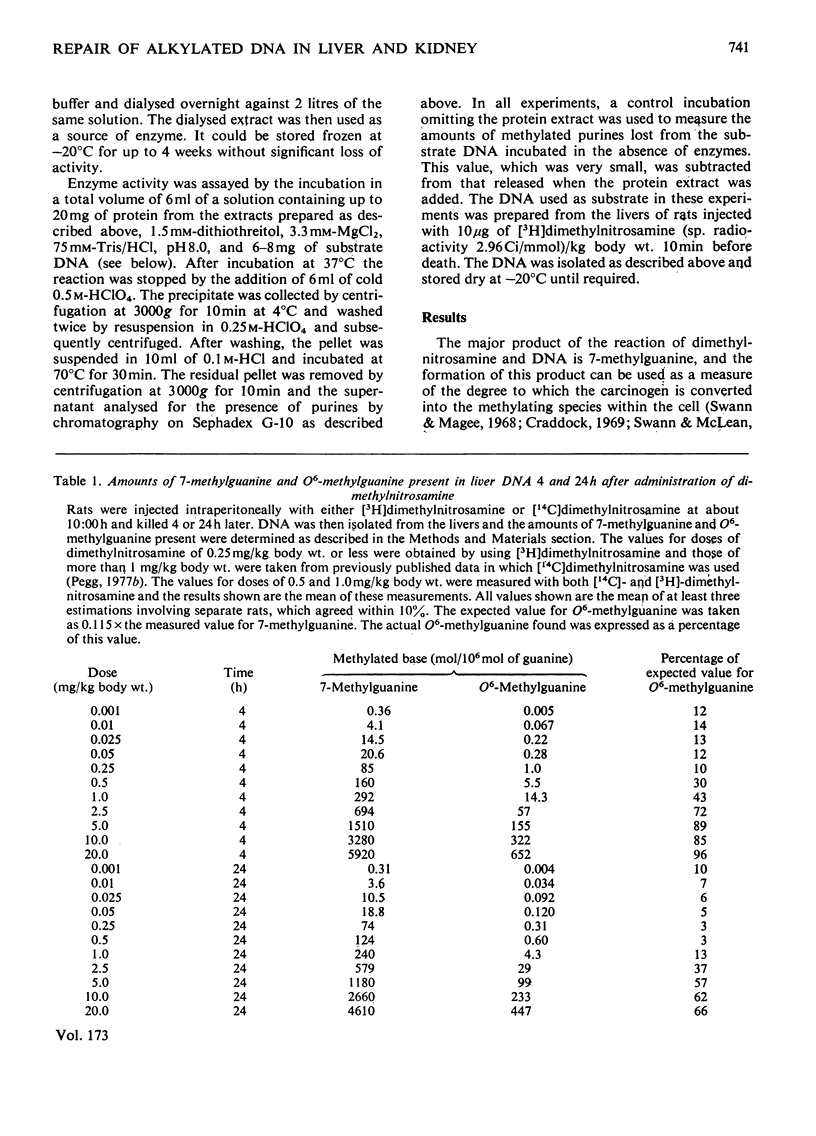
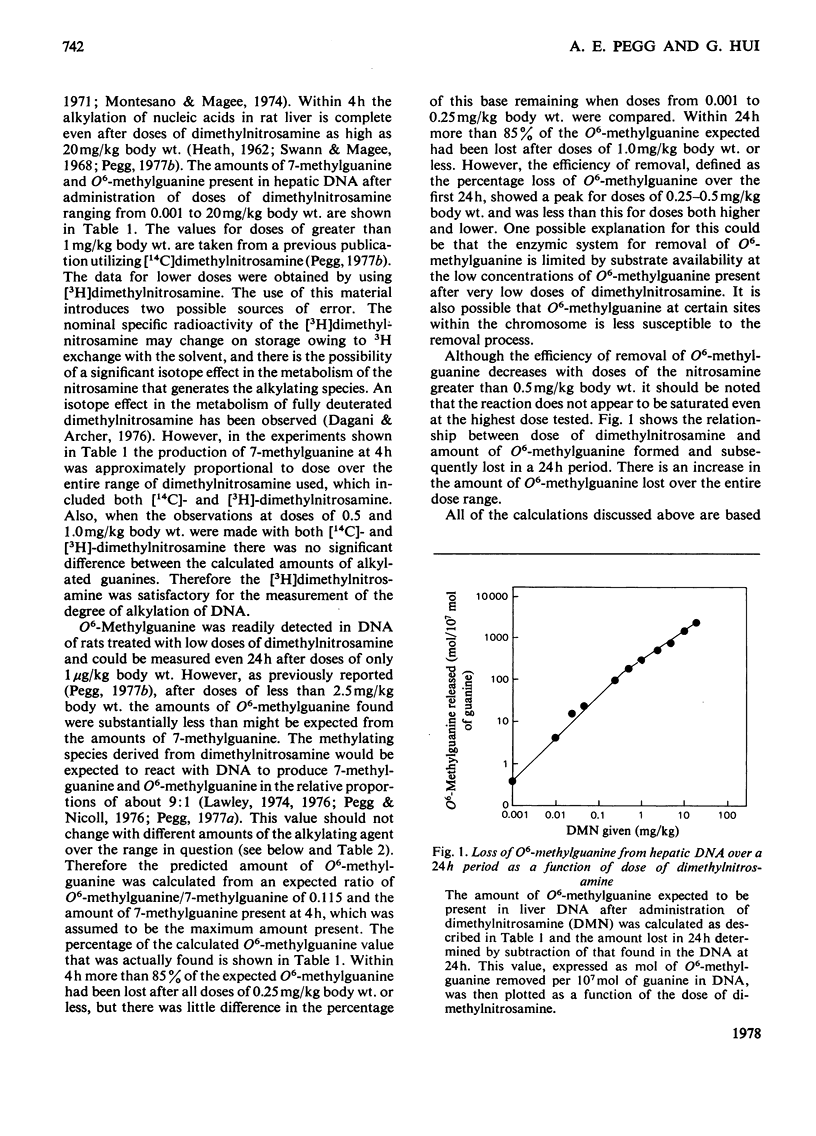
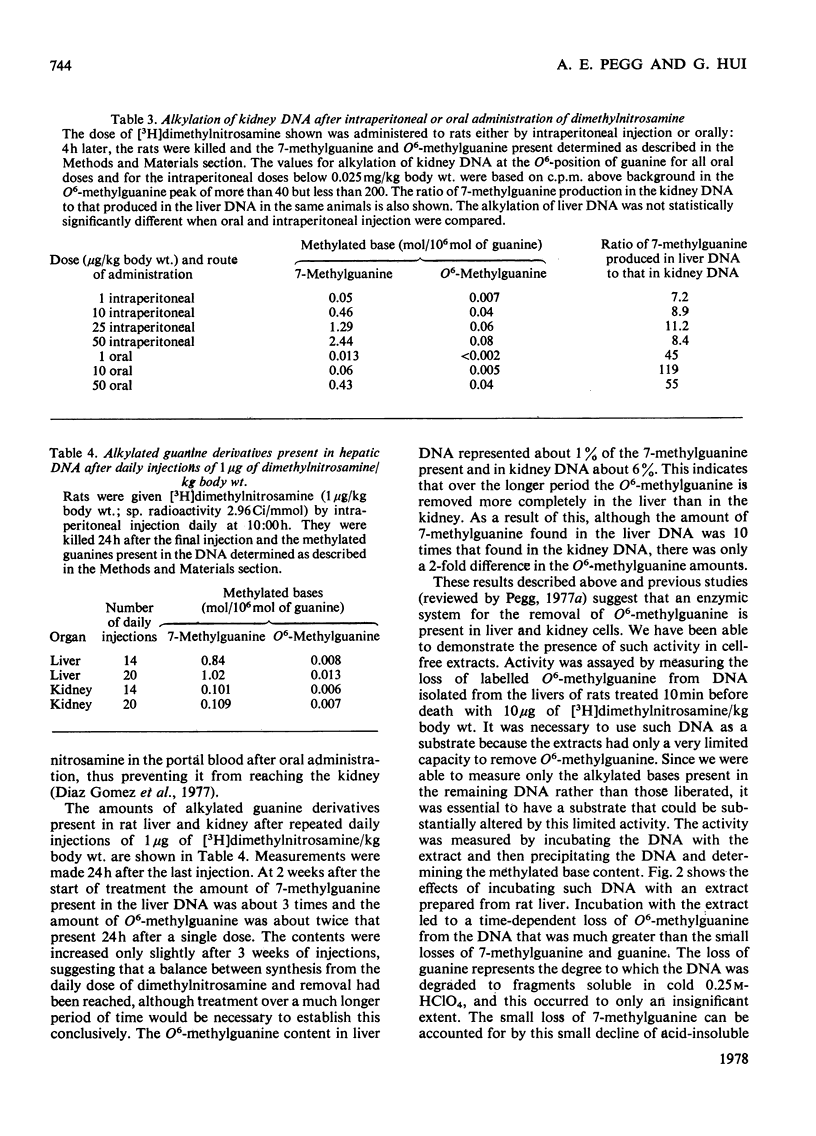
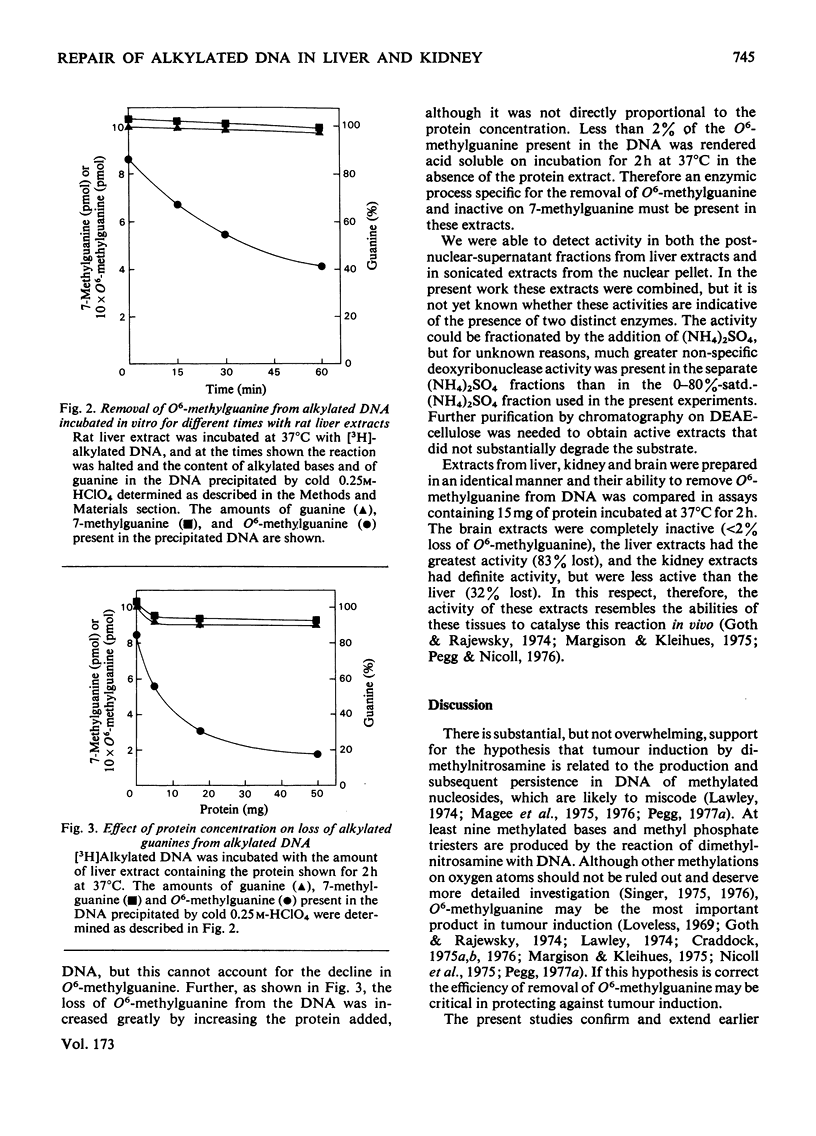
1. The amounts of 7-methylguanine and O6-methylguanine present in the DNA of liver and kidney of rats 4h and 24h after administration of low doses of dimethylnitrosamine were measured. 2. O6-Methylguanine was rapidly removed from liver DNA so that less than 15% of the expected amount (on the basis of 7-methylguanine found) was present within 4h after doses of 0.25mg/kg body wt. or less. Within 24h of administration of dimethylnitrosamine at doses of 1mg/kg or below, more than 85% of the expected amount of O6-methylguanine was removed. Removal was most efficient (defined in terms of the percentage of the O6-methylguanine formed that was subsequently lost within 24h) after doses of 0.25–0.5mg/kg body wt. At doses greater or less than this the removal was less efficient, even though the absolute amount of O6-methylguanine lost during 24h increased with the dose of dimethylnitrosamine over the entire range of doses from 0.001 to 20mg/kg body wt. 3. Alkylation of kidney DNA after intraperitoneal injections of 1–50μg of dimethylnitrosamine/kg body wt. occurred at about one-tenth the extent of alkylation of liver DNA. Removal of O6-methylguanine from the DNA also took place in the kidney, but was slower than in the liver. 4. After oral administration of these doses of dimethylnitrosamine, the alkylation of kidney DNA was much less than after intraperitoneal administration and represented only 1–2% of that found in the liver. 5. Alkylation of liver and kidney DNA was readily detectable when measured 24h after the final injection in rats that received daily injections of 1μg of [3H]dimethylnitrosamine/kg for 2 or 3 weeks. After 3 weeks, O6-methylguanine contents in the liver DNA were about 1% of the 7-methylguanine contents. The amount of 7-methylguanine in the liver DNA was 10 times that in the kidney DNA, but liver O6-methylguanine contents were only twice those in the kidney. 6. Extracts able to catalyse the removal of O6-methylguanine from alkylated DNA in vitro were isolated from liver and kidney. These extracts did not lead to the loss of 7-methylguanine from DNA. 7. The possible relevance of the formation and removal of O6-methylguanine in DNA to the risk of tumour induction by exposure to low concentrations of dimethylnitrosamine is discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer M. C., Wishnok J. S. Quantitative aspects of human exposure to nitrosamines. Food Cosmet Toxicol. 1977 Jun;15(3):233–235. doi: 10.1016/s0015-6264(77)80395-7. [DOI] [PubMed] [Google Scholar]
- Bacchetti S., Benne R. Purification and characterization of an endonuclease from calf thymus acting on irradiated DNA. Biochim Biophys Acta. 1975 May 16;390(3):285–297. doi: 10.1016/0005-2787(75)90349-4. [DOI] [PubMed] [Google Scholar]
- Brent T. P. Partial purification of endonuclease activity from human lymphoblasts. Separation of activities for depurinated DNA and DNA irradiated with ultraviolet light. Biochim Biophys Acta. 1975 Oct 1;407(2):191–199. doi: 10.1016/0005-2787(75)90284-1. [DOI] [PubMed] [Google Scholar]
- Brent T. P. Purification and characterization of human endonucleases specific for damaged DNA. Analysis of lesions induced by ultraviolet or x-radiation. Biochim Biophys Acta. 1976 Nov 12;454(1):172–183. doi: 10.1016/0005-2787(76)90363-4. [DOI] [PubMed] [Google Scholar]
- Craddock V. M. Effect of a single treatment with the alkylating carcinogens dimethylnitrosamine and methyl methanesulphonate on liver regenerating after partial hepatectomy. III. Effect on DNA synthesis in vivo and on DNA polymerase activity assayed in vitro. Chem Biol Interact. 1976 Nov;15(3):247–256. doi: 10.1016/0009-2797(76)90150-2. [DOI] [PubMed] [Google Scholar]
- Craddock V. M. Effect of a single treatment with the alkylating carcinogens dimethylnitrosamine, diethylnitrosamine and methyl methanesulphonate, on liver regenerating after partial hepatectomy. II. Alkylation of DNA and inhibition of DNA replication. Chem Biol Interact. 1975 May;10(5):323–332. doi: 10.1016/0009-2797(75)90053-8. [DOI] [PubMed] [Google Scholar]
- Craddock V. M. Stability of deoxyribonucleic acid methylated in the intact animal by administration of dimethylnitrosamine. Rate of breakdown in vivo and in vitro at different dosages. Biochem J. 1969 Feb;111(4):497–502. doi: 10.1042/bj1110497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock V. M. The pattern of methylated purines formed in DNA of intact and regenerating liver of rats treated with the carcinogen dimethylnitrosamine. Biochim Biophys Acta. 1973 Jun 23;312(2):202–210. doi: 10.1016/0005-2787(73)90365-1. [DOI] [PubMed] [Google Scholar]
- Dagani D., Archer M. C. Deuterium isotope effect in the microsomal metabolism of dimethylnitrosamine. J Natl Cancer Inst. 1976 Oct;57(4):955–957. doi: 10.1093/jnci/57.4.955. [DOI] [PubMed] [Google Scholar]
- Druckrey H., Preussmann R., Ivankovic S., Schmähl D. Organotrope carcinogene Wirkungen bei 65 verschiedenen N-Nitroso-Verbindungen an BD-Ratten. Z Krebsforsch. 1967;69(2):103–201. [PubMed] [Google Scholar]
- Fiddler The occurrence and determination of N-nitroso compounds. Toxicol Appl Pharmacol. 1975 Mar;31(3):352–360. doi: 10.1016/0041-008x(75)90256-2. [DOI] [PubMed] [Google Scholar]
- Fine D. H., Ross R., Rounbehler D. P., Silvergleid A., Song L. Formation in vivo of volatile N-nitrosamines in man after ingestion of cooked bacon and spinach. Nature. 1977 Feb 24;265(5596):753–755. doi: 10.1038/265753a0. [DOI] [PubMed] [Google Scholar]
- Fine D. H., Rounbehler D. P., Belcher N. M., Epstein S. S. N-nitroso compounds: detection in ambient air. Science. 1976 Jun 25;192(4246):1328–1330. doi: 10.1126/science.192.4246.1328. [DOI] [PubMed] [Google Scholar]
- Gomez M. I., Swann P. F., Magee P. N. The absorption and metabolism in rats of small oral doses of dimethylnitrosamine. Implication for the possible hazard of dimethylnitrosamine in human food. Biochem J. 1977 Jun 15;164(3):497–500. doi: 10.1042/bj1640497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goth-Goldstein R. Repair of DNA damaged by alkylating carcinogens is defective in xeroderma pigmentosum-derived fibroblasts. Nature. 1977 May 5;267(5606):81–82. doi: 10.1038/267081a0. [DOI] [PubMed] [Google Scholar]
- Goth R., Rajewsky M. F. Persistence of O6-ethylguanine in rat-brain DNA: correlation with nervous system-specific carcinogenesis by ethylnitrosourea. Proc Natl Acad Sci U S A. 1974 Mar;71(3):639–643. doi: 10.1073/pnas.71.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman L., Braun A., Feldberg R., Mahler I. Enzymatic repair of DNA. Annu Rev Biochem. 1975;44:19–43. doi: 10.1146/annurev.bi.44.070175.000315. [DOI] [PubMed] [Google Scholar]
- Hashimoto S., Yokokura T., Kawai Y., Mutai M. Dimethylnitrosamine formation in the gastro-intestinal tract of rats. Food Cosmet Toxicol. 1976 Dec;14(6):553–556. doi: 10.1016/s0015-6264(76)80007-7. [DOI] [PubMed] [Google Scholar]
- Heath D. F. The decomposition and toxicity of dialkylnitrosamines in rats. Biochem J. 1962 Oct;85(1):72–91. doi: 10.1042/bj0850072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirtikar D. M., Cathcart G. R., Goldthwait D. A. Endonuclease II, apurinic acid endonuclease, and exonuclease III. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4324–4328. doi: 10.1073/pnas.73.12.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirtikar D. M., Goldthwait D. A. The enzymatic release of O6-methylguanine and 3-methyladenine from DNA reacted with the carcinogen N-methyl-N-nitrosourea. Proc Natl Acad Sci U S A. 1974 May;71(5):2022–2026. doi: 10.1073/pnas.71.5.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleihues P., Margison G. P. Exhaustion and recovery of repair excision of O6-methylguanine from rat liver DNA. Nature. 1976 Jan 15;259(5539):153–155. doi: 10.1038/259153a0. [DOI] [PubMed] [Google Scholar]
- Kuebler J. P., Goldthwait D. A. An endonuclease from calf liver specific for apurinic sites in DNA. Biochemistry. 1977 Apr 5;16(7):1370–1377. doi: 10.1021/bi00626a021. [DOI] [PubMed] [Google Scholar]
- Kuhnlein U., Penhoet E. E., Linn S. An altered apurinic DNA endonuclease activity in group A and group D xeroderma pigmentosum fibroblasts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1169–1173. doi: 10.1073/pnas.73.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laval J. Two enzymes are required from strand incision in repair of alkylated DNA. Nature. 1977 Oct 27;269(5631):829–832. doi: 10.1038/269829a0. [DOI] [PubMed] [Google Scholar]
- Lawley P. D., Orr D. J. Specific excision of methylation products from DNA of Escherichia coli treated with N-methyl-N'-nitro-N-nitrosoguanidine. Chem Biol Interact. 1970 Aug;2(2):154–157. doi: 10.1016/0009-2797(70)90047-5. [DOI] [PubMed] [Google Scholar]
- Lawley P. D., Shah S. A. Methylation of ribonucleic acid by the carcinogens dimethyl sulphate, N-methyl-N-nitrosourea and N-methyl-N'-nitro-N-nitrosoguanidine. Comparisons of chemical analyses at the nucleoside and base levels. Biochem J. 1972 Jun;128(1):117–132. doi: 10.1042/bj1280117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley P. D. Some chemical aspects of dose-response relationships in alkylation mutagenesis. Mutat Res. 1974 Jun;23(3):283–295. doi: 10.1016/0027-5107(74)90102-x. [DOI] [PubMed] [Google Scholar]
- Lawley P. D., Thatcher C. J. Methylation of deoxyribonucleic acid in cultured mammalian cells by N-methyl-N'-nitro-N-nitrosoguanidine. The influence of cellular thiol concentrations on the extent of methylation and the 6-oxygen atom of guanine as a site of methylation. Biochem J. 1970 Feb;116(4):693–707. doi: 10.1042/bj1160693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley P. D., Warren W. Specific excision of ethylated purines from DNA of Escherichia coli treated with N-ethyl-N-nitrosourea. Chem Biol Interact. 1975 Jul;11(1):55–57. doi: 10.1016/0009-2797(75)90066-6. [DOI] [PubMed] [Google Scholar]
- Lijinsky W., Epstein S. S. Nitrosamines as environmental carcinogens. Nature. 1970 Jan 3;225(5227):21–23. doi: 10.1038/225021a0. [DOI] [PubMed] [Google Scholar]
- Lindahl T. New class of enzymes acting on damaged DNA. Nature. 1976 Jan 1;259(5538):64–66. doi: 10.1038/259064a0. [DOI] [PubMed] [Google Scholar]
- Linsley W. S., Penhoet E. E., Linn S. Human endonuclease specific for apurinic/apyrimidinic sites in DNA. Partial purification and characterization of multiple forms from placenta. J Biol Chem. 1977 Feb 25;252(4):1235–1242. [PubMed] [Google Scholar]
- Ljungquist S., Andersson A., Lindahl T. A mammalian endonuclease specific for apurinic sites in double-stranded deoxyribonucleic acid. II. Further studies on the substrate specificity. J Biol Chem. 1974 Mar 10;249(5):1536–1540. [PubMed] [Google Scholar]
- Loveless A. Possible relevance of O-6 alkylation of deoxyguanosine to the mutagenicity and carcinogenicity of nitrosamines and nitrosamides. Nature. 1969 Jul 12;223(5202):206–207. doi: 10.1038/223206a0. [DOI] [PubMed] [Google Scholar]
- Magee P. N., Barnes J. M. Carcinogenic nitroso compounds. Adv Cancer Res. 1967;10:163–246. doi: 10.1016/s0065-230x(08)60079-2. [DOI] [PubMed] [Google Scholar]
- Margison G. P., Capps M. J., O'Connor P. J., Craig A. W. Loss of 7-methylguanine from rat liver DNA after methylation in vivo with methylmethanesulphonate or dimethylnitrosamine. Chem Biol Interact. 1973 Feb;6(2):119–124. doi: 10.1016/0009-2797(73)90078-1. [DOI] [PubMed] [Google Scholar]
- Margison G. P., Kleihues P. Chemical carcinogenesis in the nervous system. Preferential accumulation of O6-methylguanine in rat brain deoxyribonucleic acid during repetitive administration of N-methyl-N-nitrosourea. Biochem J. 1975 Jun;148(3):521–525. doi: 10.1042/bj1480521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margison G. P., Margison J. M., Montesano R. Accumulation of O6-methylguanine in non-target-tissue deoxyribonucleic acid during chronic administration of dimethylnitrosamine. Biochem J. 1977 Sep 1;165(3):463–468. doi: 10.1042/bj1650463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margison G. P., Margison J. M., Montesano R. Methylated purines in the deoxyribonucleic acid of various Syrian-golden-hamster tissues after administration of a hepatocarcinogenic dose of dimethylnitrosamine. Biochem J. 1976 Sep 1;157(3):627–634. doi: 10.1042/bj1570627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. T., Lawley P. D., Shah S. A. Cellular reactions of O6-methylguanine, a product of some alkylating carcinogens. Biochem J. 1973 Oct;136(2):387–393. doi: 10.1042/bj1360387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirvish S. S. Formation of N-nitroso compounds: chemistry, kinetics, and in vivo occurrence. Toxicol Appl Pharmacol. 1975 Mar;31(3):325–351. doi: 10.1016/0041-008x(75)90255-0. [DOI] [PubMed] [Google Scholar]
- Mortelmans K., Friedberg E. C., Slor H., Thomas G., Cleaver J. E. Defective thymine dimer excision by cell-free extracts of xeroderma pigmentosum cells. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2757–2761. doi: 10.1073/pnas.73.8.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto N., Takayama S. Rapid loss of 7-methylguanine from liver nucleic acids in mice during the initial stage of liver carcinogenesis induced by dimethylnitrosamine. Biochem Biophys Res Commun. 1974 May 7;58(1):242–249. doi: 10.1016/0006-291x(74)90918-8. [DOI] [PubMed] [Google Scholar]
- Nicoll J. W., Swann P. F., Pegg A. E. Effect of dimethylnitrosamine on persistence of methylated guanines in rat liver and kidney DNA. Nature. 1975 Mar 20;254(5497):261–262. doi: 10.1038/254261a0. [DOI] [PubMed] [Google Scholar]
- O'Connor P. J., Capps M. J., Craig A. W. Comparative studies of the hepatocarcinogen N,N-dimethylnitrosamine in vivo: reaction sites in rat liver DNA and the significance of their relative stabilities. Br J Cancer. 1973 Feb;27(2):153–166. doi: 10.1038/bjc.1973.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E. Alkylation of rat liver DNA by dimethylnitrosamine: effect of dosage on O6-methylguanine levels. J Natl Cancer Inst. 1977 Mar;58(3):681–687. doi: 10.1093/jnci/58.3.681. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Formation and metabolism of alkylated nucleosides: possible role in carcinogenesis by nitroso compounds and alkylating agents. Adv Cancer Res. 1977;25:195–269. doi: 10.1016/s0065-230x(08)60635-1. [DOI] [PubMed] [Google Scholar]
- Regan J. D., Setlow R. B. Two forms of repair in the DNA of human cells damaged by chemical carcinogens and mutagens. Cancer Res. 1974 Dec;34(12):3318–3325. [PubMed] [Google Scholar]
- Samson L., Cairns J. A new pathway for DNA repair in Escherichia coli. Nature. 1977 May 19;267(5608):281–283. doi: 10.1038/267281a0. [DOI] [PubMed] [Google Scholar]
- Singer B. All oxygens in nucleic acids react with carcinogenic ethylating agents. Nature. 1976 Nov 25;264(5584):333–339. doi: 10.1038/264333a0. [DOI] [PubMed] [Google Scholar]
- Singer B. The chemical effects of nucleic acid alkylation and their relation to mutagenesis and carcinogenesis. Prog Nucleic Acid Res Mol Biol. 1975;15(0):219–284. [PubMed] [Google Scholar]
- Swann P. F., Magee P. N. Nitrosamine-induced carcinogenesis. The alklylation of nucleic acids of the rat by N-methyl-N-nitrosourea, dimethylnitrosamine, dimethyl sulphate and methyl methanesulphonate. Biochem J. 1968 Nov;110(1):39–47. doi: 10.1042/bj1100039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann P. F., McLean A. E. Cellular injury and carcinogenesis. The effect of a protein-free high-carbohydrate diet on the metabolism of dimethylnitrosamine in the rat. Biochem J. 1971 Sep;124(2):283–288. doi: 10.1042/bj1240283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teebor G. W., Duker N. J., Becker F. F. Normal endonuclease activities for damaged DNA during hepatocarcinogenesis. Biochim Biophys Acta. 1977 Jul 15;477(2):125–131. doi: 10.1016/0005-2787(77)90228-3. [DOI] [PubMed] [Google Scholar]
- Teebor G. W., Duker N. J. Human endonuclease activity for DNA apurinic sites. Nature. 1975 Dec 11;258(5535):544–547. doi: 10.1038/258544a0. [DOI] [PubMed] [Google Scholar]
- Terracini B., Magee P. N., Barnes J. M. Hepatic pathology in rats on low dietary levels of dimethylnitrosamine. Br J Cancer. 1967 Sep;21(3):559–565. doi: 10.1038/bjc.1967.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verly W. G., Paquette Y. An endonuclease for depurinated DNA in rat liver. Can J Biochem. 1973 Jul;51(7):1003–1009. doi: 10.1139/o73-130. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lancker J. L., Tomura T. Purification and some properties of a mammalian repair endonuclease. Biochim Biophys Acta. 1974 Jun 14;353(1):99–114. doi: 10.1016/0005-2787(74)90101-4. [DOI] [PubMed] [Google Scholar]


