Abstract
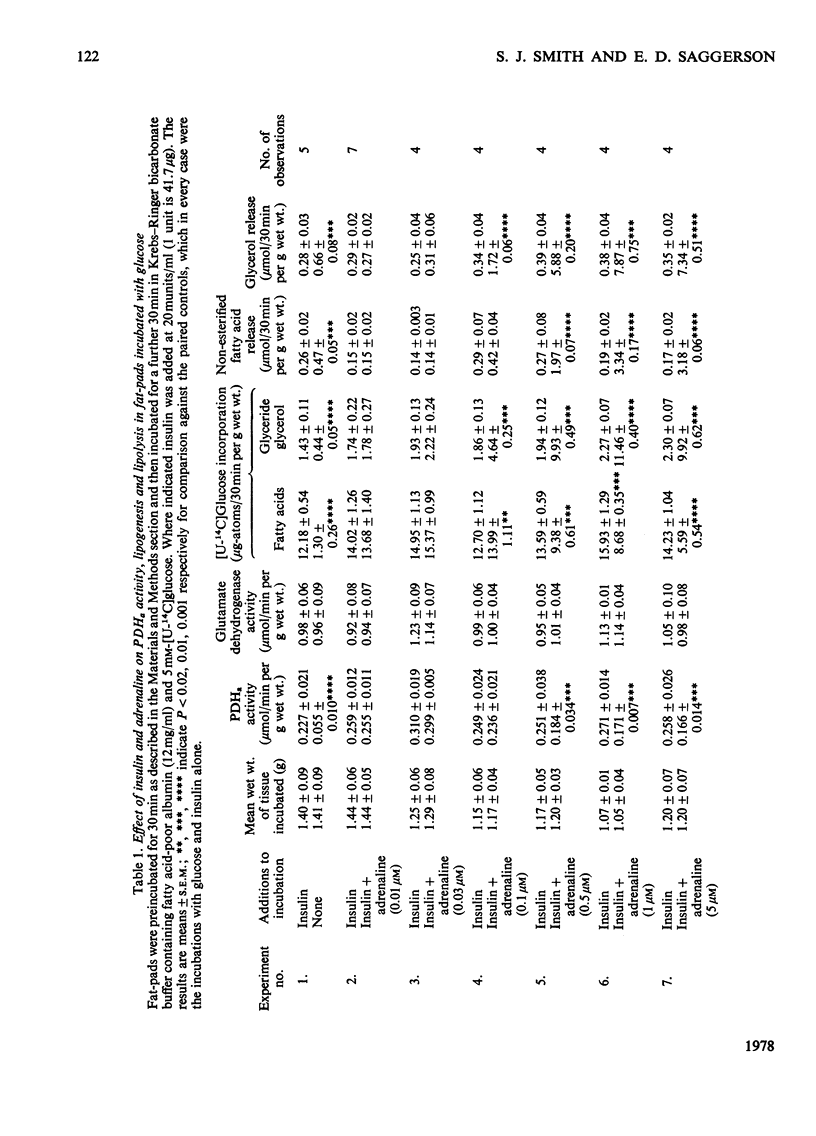
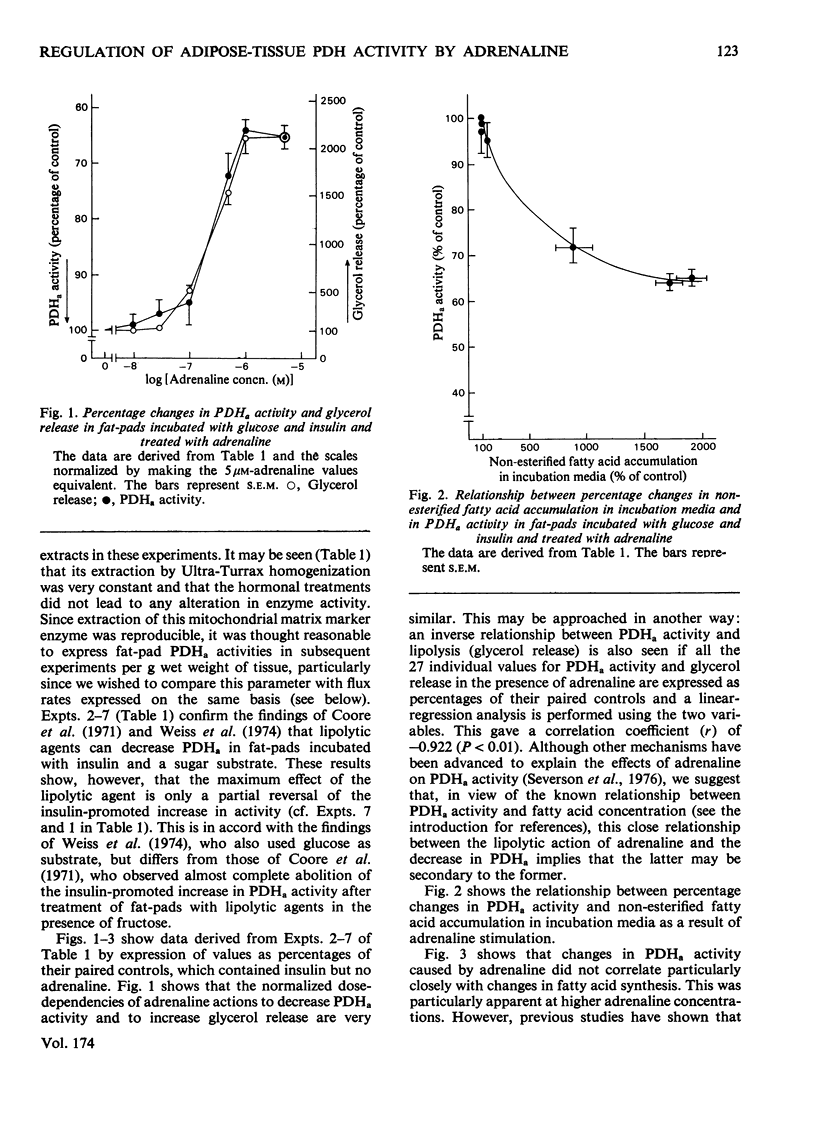
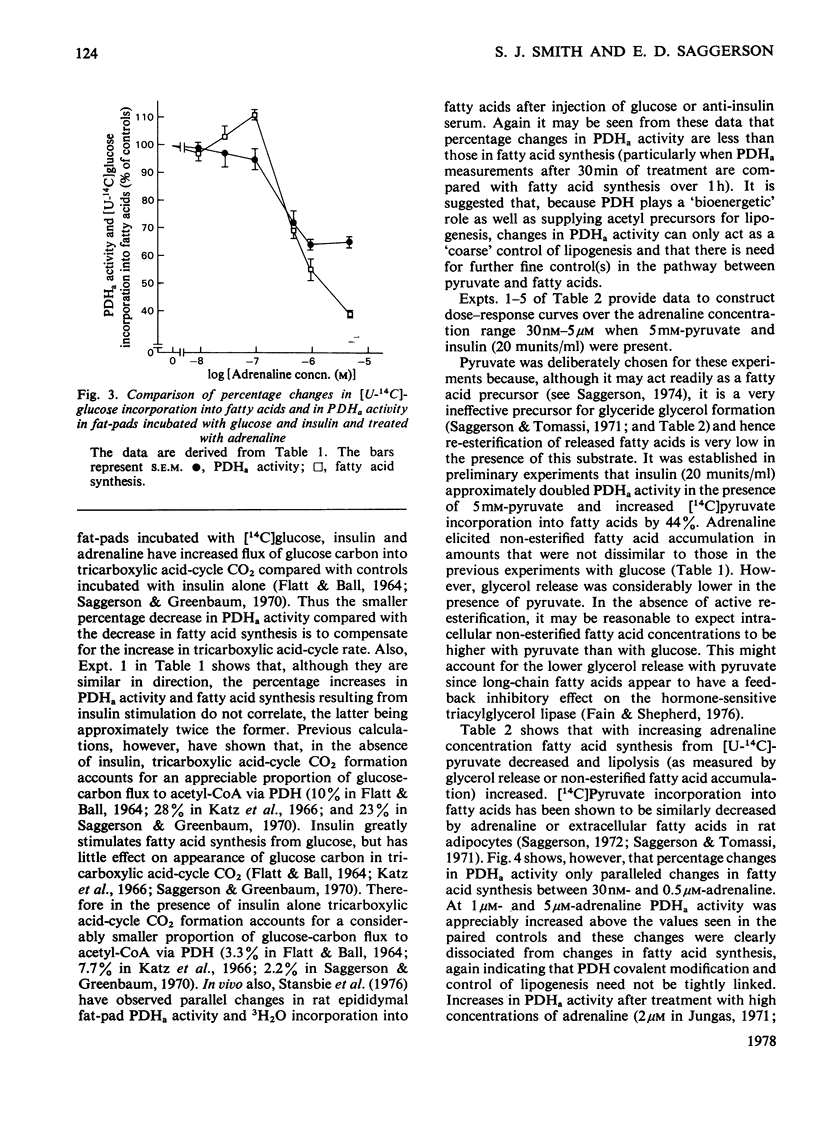
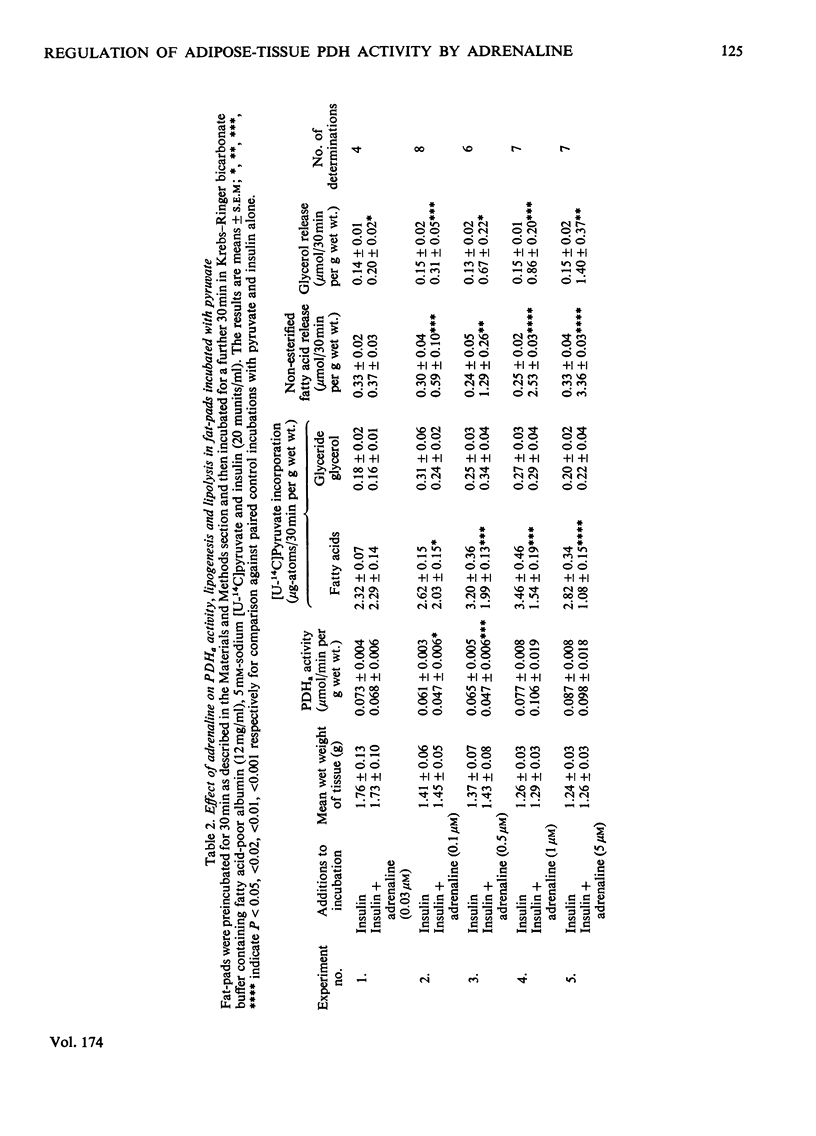
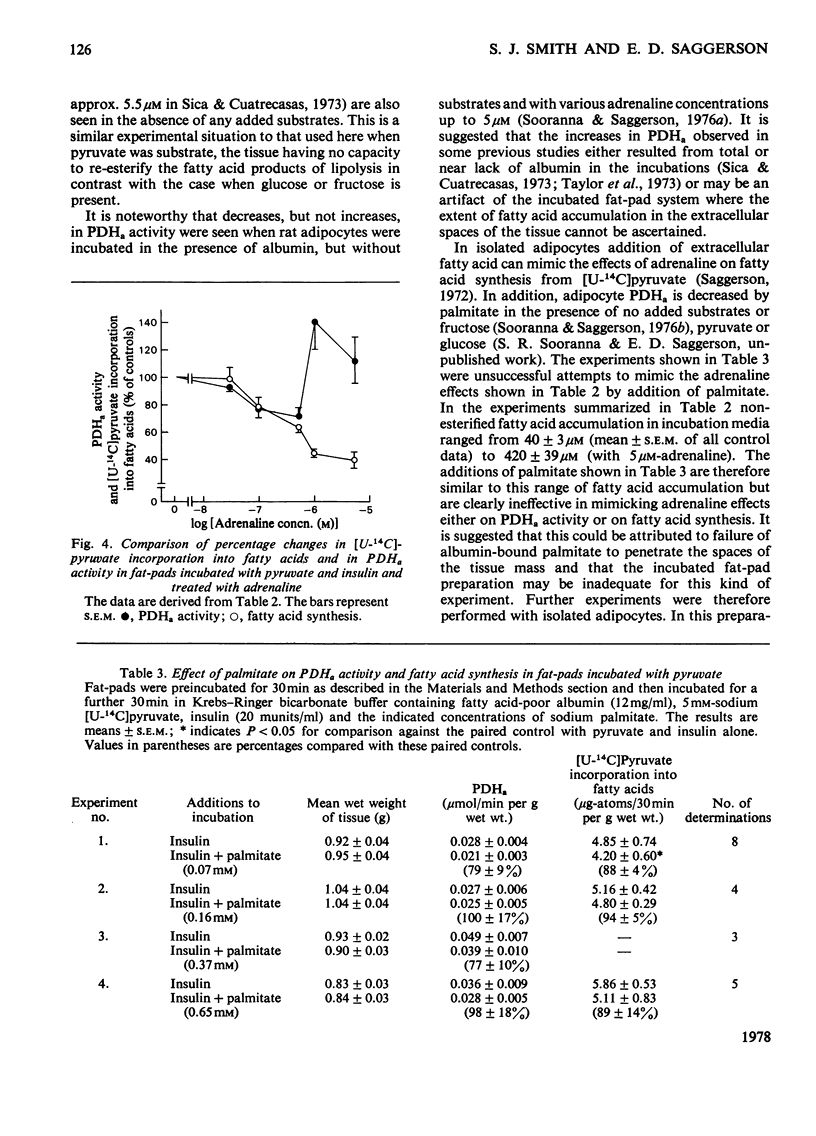
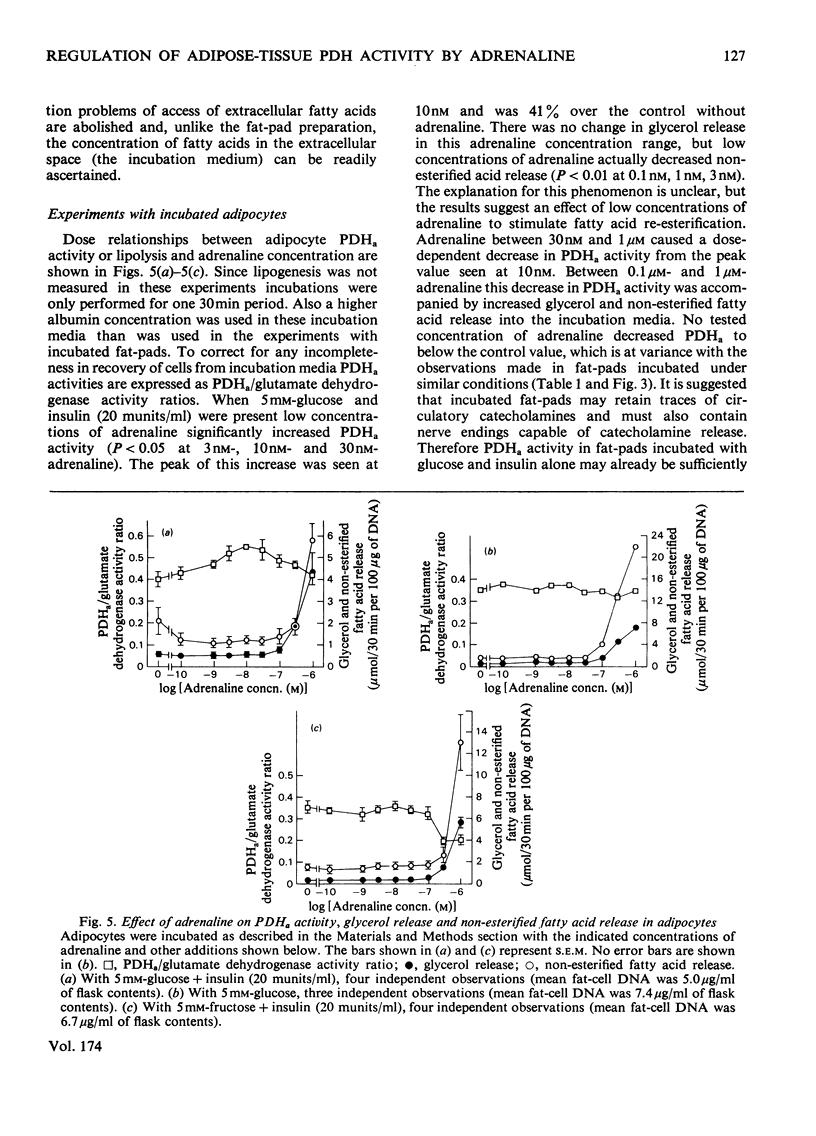
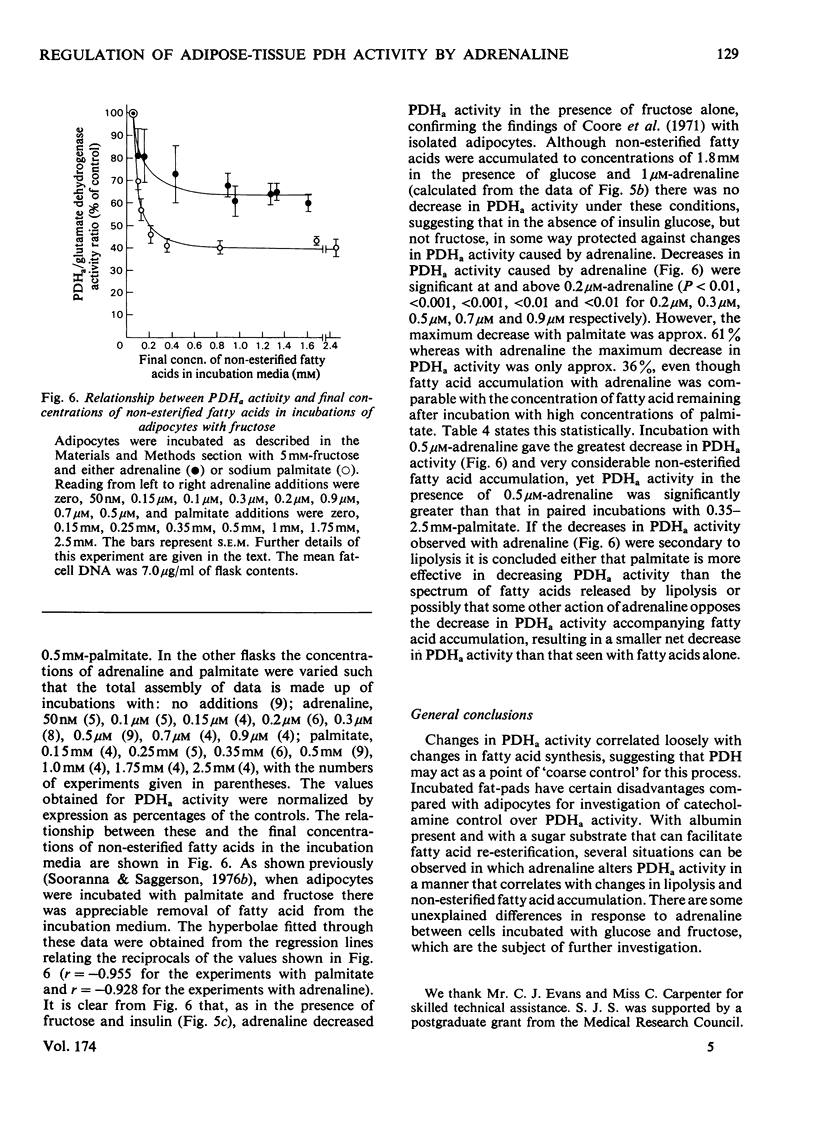
1. Dose-dependent effects of adrenaline on PDHa activity were investigated with both incubated rat epidiymal fat-pads and isolated adipocytes. 2. Adrenaline (10nM- 5 micrometer) decreased PDHa activity in fat-pads incubated with 5 mM-[U-14C]glucose + insulin (20 munits/ml). Changes in [U-14C]glucose incorporation into fatty acids in these tissues correlated only loosely with changes in PDHa activity. There was a good inverse relationship between adrenaline-induced changes in PDHa activity and increases in lipolysis (glycerol release). 3. Adrenaline (10nM - 0.5 micrometer) decreased PDHa activity in fat-pads incubated with 5 mM-[U-14C]pyruvate + insulin (20 munits/ml), whereas 1 micrometer- and 5 micrometer-adrenaline slightly increased PDHa activity. All concentrations of adrenaline tested decreased [U-14C]pyruvate incorporation into fatty acids. Between 10nM- and 0.5 micrometer-adrenaline percentage decreases in PDHa activity paralleled decreases in faty acid synthesis. 4. Effects of adrenaline on PDHa activity and fatty acid synthesis in fat-pads incubated with 5mM-[U-14C]pyruvate + insulin (20 munits/ml) could not be mimicked by addition of albumin-bound palmitate. 5. The response of PDHa activity to adrenaline (0.1 nM - 1 micrometer) in isolated adipocytes differed with the carbohydrate substrate used in the incubations. With 5 mM-glucose + insulin (20 munits/ml), PDHa activity was significantly increased by 10 nM-adrenaline, but not by 1 micrometer-adrenaline, the response to adrenaline being biphasic. There was some correlation between PDHa activity and accumulation of non-esterified fatty acids. With 5 mM-glucose alone adrenaline (0.1 nM - 1 micrometer) had no effect on PDHa activity even though lipolysis was increased by adrenaline (0.1 micrometer - 1 micrometer). With 5mM-fructose in the presence and absence of insulin, lipolytic doses of adrenaline decreased PDHa activity. No tested concentrations of adrenaline increased PDHa with this substrate. 6. In the presence of 5 mM-fructose, palmitate was significantly more effective than adrenaline with respect to the maximum decrease in PDHa activity that could be elicited. 4. The relationship of changes in PDHa activity to changes in lipogenesis and the likelihood of adrenaline-induced changes in PDHa activity being secondary to changes in non-esterified fatty acid metabolism are discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Coore H. G., Denton R. M., Martin B. R., Randle P. J. Regulation of adipose tissue pyruvate dehydrogenase by insulin and other hormones. Biochem J. 1971 Nov;125(1):115–127. doi: 10.1042/bj1250115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coore H. G., Field B. Properties of pyruvate dehydrogenase of rat mammary tissue and its changes during pregnancy, lactation and weaning. Biochem J. 1974 Jul;142(1):87–95. doi: 10.1042/bj1420087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOLE V. P. A relation between non-esterified fatty acids in plasma and the metabolism of glucose. J Clin Invest. 1956 Feb;35(2):150–154. doi: 10.1172/JCI103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS W. H., MUELLER P. S. EFFECTS OF PALMITATE ON THE METABOLISM OF LEUKOCYTES FROM GUINEA PIG EXUDATE. J Lipid Res. 1963 Jan;4:39–45. [PubMed] [Google Scholar]
- FLATT J. P., BALL E. G. STUDIES ON THE METABOLISM OF ADIPOSE TISSUE. XV. AN EVALUATION OF THE MAJOR PATHWAYS OF GLUCOSE CATABOLISM AS INFLUENCED BY INSULIN AND EPINEPHRINE. J Biol Chem. 1964 Mar;239:675–685. [PubMed] [Google Scholar]
- Fain J. N., Shephard R. E. Inhibition of adenosine 3':k'-monophosphate accumulation white fat acids, lactate, and beta-hydroxybutyrate. J Lipid Res. 1976 Jul;17(4):377–385. [PubMed] [Google Scholar]
- GARLAND P. B., RANDLE P. J. A rapid enzymatic assay for glycerol. Nature. 1962 Dec 8;196:987–988. doi: 10.1038/196987a0. [DOI] [PubMed] [Google Scholar]
- Guder W. G., Wieland O. H. Metabolism of isolated kidney tubules. Regulation of pyruvate dehydrogenase by metabolic substrates. Eur J Biochem. 1974 Mar 1;42(2):529–538. doi: 10.1111/j.1432-1033.1974.tb03368.x. [DOI] [PubMed] [Google Scholar]
- Himms-Hagen J. Adrenergic receptors for metabolic responses in adipose tissue. Fed Proc. 1970 Jul-Aug;29(4):1388–1401. [PubMed] [Google Scholar]
- ITAYA K., UI M. COLORIMETRIC DETERMINATION OF FREE FATTY ACIDS IN BIOLOGICAL FLUIDS. J Lipid Res. 1965 Jan;6:16–20. [PubMed] [Google Scholar]
- Jungas R. L. Effect of insulin on fatty acid ynthesis from pyruvate, lactage, or endogenous sources in adipose tissue: evidence for the hormonal regulation of pyruvate dehydrogenase. Endocrinology. 1970 Jun;86(6):1368–1375. doi: 10.1210/endo-86-6-1368. [DOI] [PubMed] [Google Scholar]
- Jungas R. L. Hormonal regulation of pyruvate dehydrogenase. Metabolism. 1971 Jan;20(1):43–53. doi: 10.1016/0026-0495(71)90058-8. [DOI] [PubMed] [Google Scholar]
- Katz J., Landau B. R., Bartsch G. E. The pentose cycle, triose phosphate isomerization, and lipogenesis in rat adipose tissue. J Biol Chem. 1966 Feb 10;241(3):727–740. [PubMed] [Google Scholar]
- Martin B. R., Denton R. M., Pask H. T., Randle P. J. Mechanisms regulating adipose-tissue pyruvate dehydrogenase. Biochem J. 1972 Sep;129(3):763–773. doi: 10.1042/bj1290763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B. R., Denton R. M. The intracellular localization of enzymes in white-adipose-tissue fat-cells and permeability properties of fat-cell mitochondria. Transfer of acetyl units and reducing power between mitochondria and cytoplasm. Biochem J. 1970 May;117(5):861–877. doi: 10.1042/bj1170861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry M. C., Hales C. N. Factors affecting the permeability of isolated fat-cells from the rat to [42K] potassium and [36Cl] chloride ions. Biochem J. 1970 Apr;117(3):615–621. doi: 10.1042/bj1170615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Saggerson E. D., Greenbaum A. L. The regulation of triglyceride synthesis and fatty acid synthesis in rat epididymal adipose tissue. Biochem J. 1970 Sep;119(2):193–219. doi: 10.1042/bj1190193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D. Lipogenesis in rat and guinea-pig isolated epididymal fat-cells. Biochem J. 1974 May;140(2):211–224. doi: 10.1042/bj1400211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D. The regulation of glyceride synthesis in isolated white-fat cells. The effects of palmitate and lipolytic agents. Biochem J. 1972 Aug;128(5):1057–1067. doi: 10.1042/bj1281057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Tomassi G. The regulation of glyceride synthesis from pyruvate in isolated fat cells. The effects of palmitate and alteration of dietary status. Eur J Biochem. 1971 Nov 11;23(1):109–117. doi: 10.1111/j.1432-1033.1971.tb01597.x. [DOI] [PubMed] [Google Scholar]
- Severson D. L., Denton R. M., Bridges B. J., Randle P. J. Exchangeable and total calcium pools in mitochondria of rat epididymal fat-pads and isolated fat-cells. Role in the regulation of pyruvate dehydrogenase activity. Biochem J. 1976 Jan 15;154(1):209–223. doi: 10.1042/bj1540209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sica V., Cuatrecasas P. Effects of insulin, epinephrine, and cyclic adenosine monophosphate on pyruvate dehydrogenase of adipose tissue. Biochemistry. 1973 Jun 5;12(12):2282–2291. doi: 10.1021/bi00736a016. [DOI] [PubMed] [Google Scholar]
- Sooranna S. R., Saggerson E. D. Interacting actions of insulin and unesterified fatty acids on lipogenic enzymes in rat adipocytes. FEBS Lett. 1976 Oct 15;69(1):144–148. doi: 10.1016/0014-5793(76)80672-2. [DOI] [PubMed] [Google Scholar]
- Sooranna S. R., Saggerson E. D. Interactions of insulin and adrenaline with glycerol phosphate acylation processes in fat-cells from rat. FEBS Lett. 1976 Apr 15;64(1):36–39. doi: 10.1016/0014-5793(76)80242-6. [DOI] [PubMed] [Google Scholar]
- Stansbie D., Brownsey R. W., Crettaz M., Denton R. M. Acute effects in vivo of anti-insulin serum on rates of fatty acid synthesis and activities of acetyl-coenzyme A carboxylase and pyruvate dehydrogenase in liver and epididymal adipose tissue of fed rats. Biochem J. 1976 Nov 15;160(2):413–416. doi: 10.1042/bj1600413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. I., Jungas R. L. Regulation of lipogenesis in adipose tissue: the significance of the activation of pyruvate dehydrogenase by insulin. Arch Biochem Biophys. 1974 Sep;164(1):12–19. doi: 10.1016/0003-9861(74)90002-2. [DOI] [PubMed] [Google Scholar]
- Taylor S. I., Mukherjee C., Jungas R. L. Studies on the mechanism of activation of adipose tissue pyruvate dehydrogenase by insulin. J Biol Chem. 1973 Jan 10;248(1):73–81. [PubMed] [Google Scholar]
- WIELAND O. Eine enzymatische Methode zur Bestimmung von Glycerin. Biochem Z. 1957;329(4):313–319. [PubMed] [Google Scholar]
- Weiss L., Löffler G., Schirmann A., Wieland O. Control of pyruvate dehydrogenase interconversion in adipose tissue by insulin. FEBS Lett. 1971 Jun 24;15(3):229–231. doi: 10.1016/0014-5793(71)80318-6. [DOI] [PubMed] [Google Scholar]
- Weiss L., Löffler G., Wieland O. H. Regulation by insulin of adipose tissue pyruvate dehydrogenase. A mechanism controlling fatty acid synthesis from carbohydrates. Hoppe Seylers Z Physiol Chem. 1974 Mar;355(3):363–377. doi: 10.1515/bchm2.1974.355.1.363. [DOI] [PubMed] [Google Scholar]
- Wieland O. H., Patzelt C., Löffler G. Active and inactive forms of pyruvate dehydrogenase in rat liver. Effect of starvation and refeeding and of insulin treatment on pyruvate-dehydrogenase interconversion. Eur J Biochem. 1972 Apr 11;26(3):426–433. doi: 10.1111/j.1432-1033.1972.tb01783.x. [DOI] [PubMed] [Google Scholar]
- Wieland O., Siess E., Schulze-Wethmar F. H., von Funcke H. G., Winton B. Active and inactive forms of pyruvate dehydrogenase in rat heart and kidney: effect of diabetes, fasting, and refeeding on pyruvate dehydrogenase interconversion. Arch Biochem Biophys. 1971 Apr;143(2):593–601. doi: 10.1016/0003-9861(71)90244-x. [DOI] [PubMed] [Google Scholar]


