Abstract
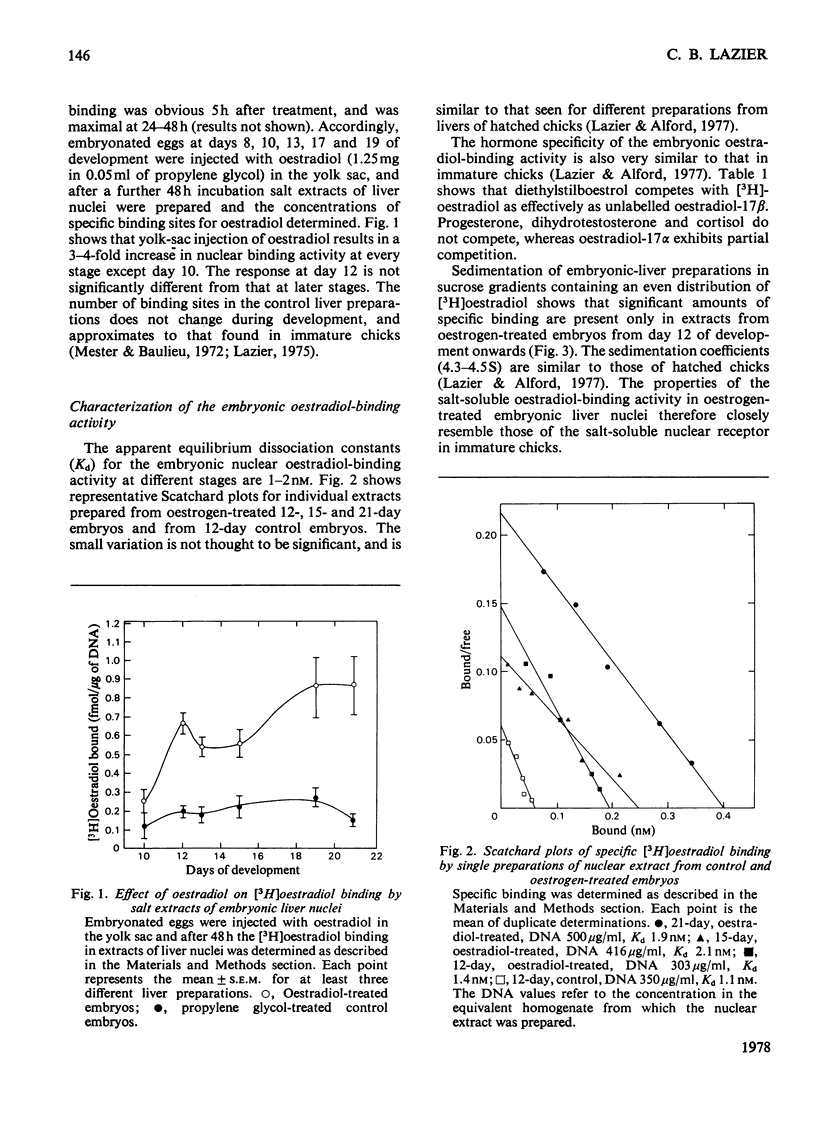
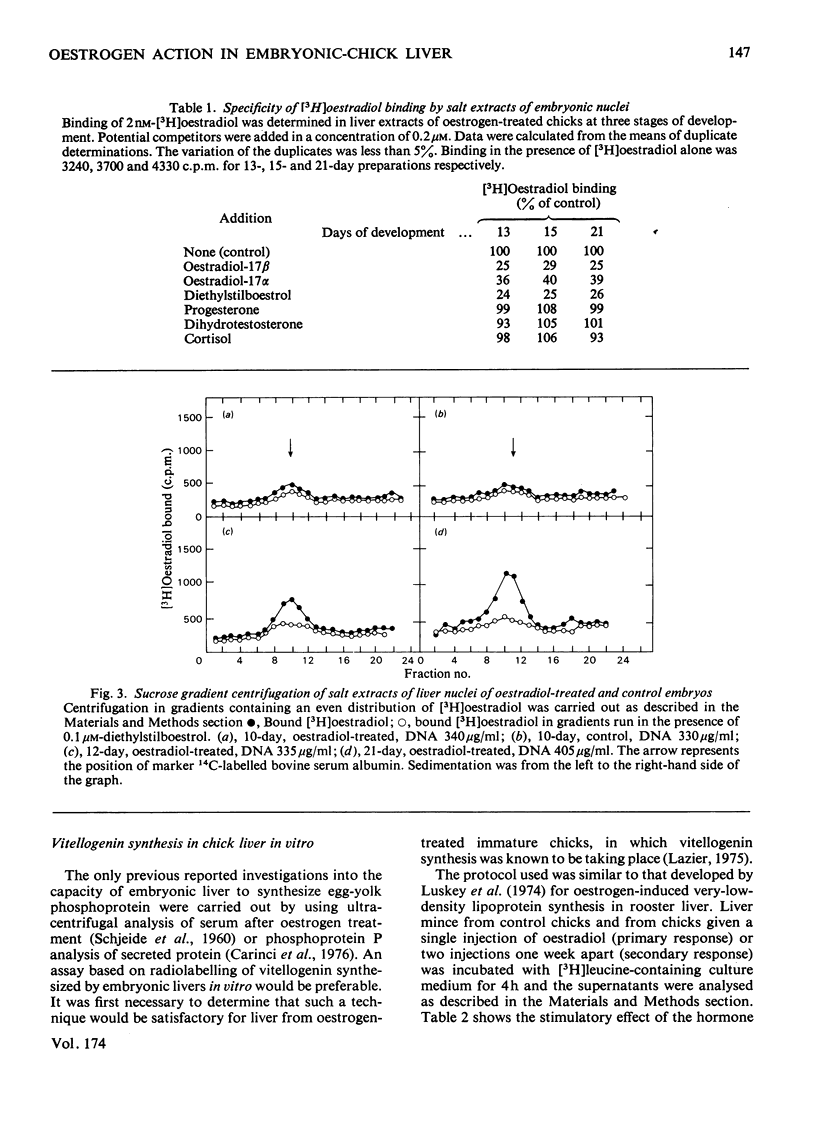
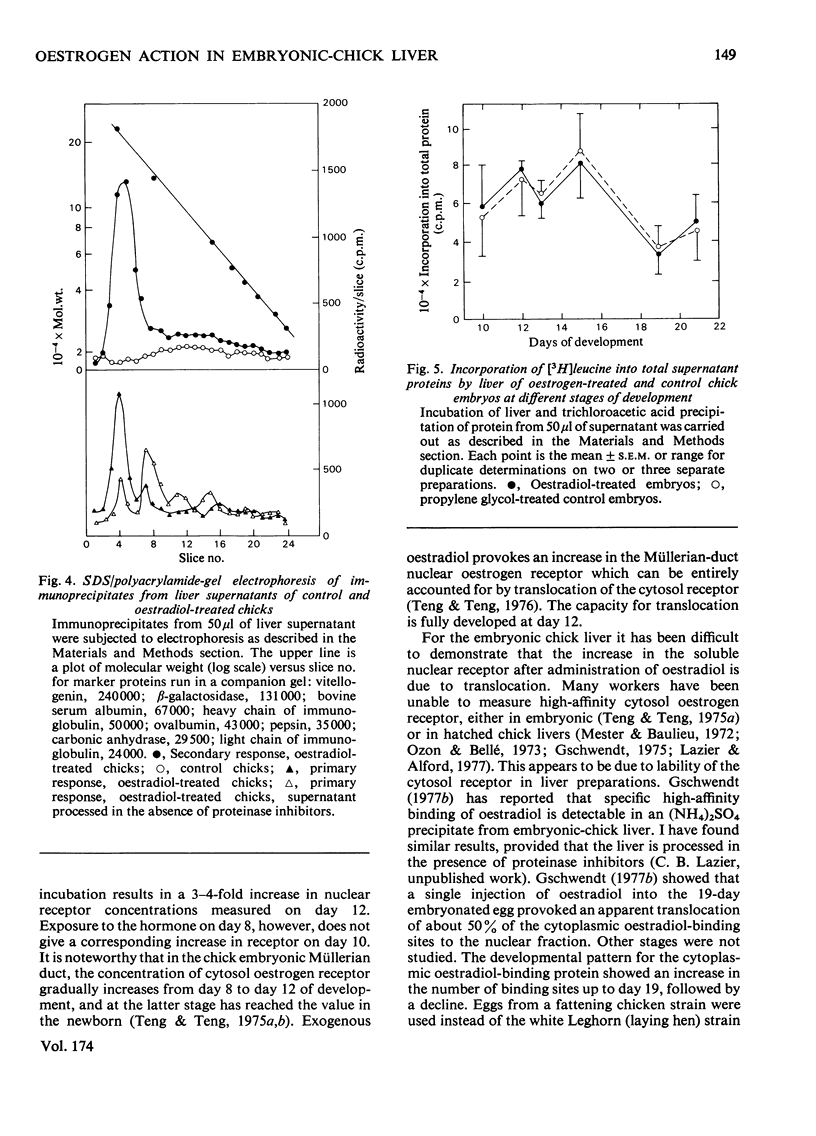
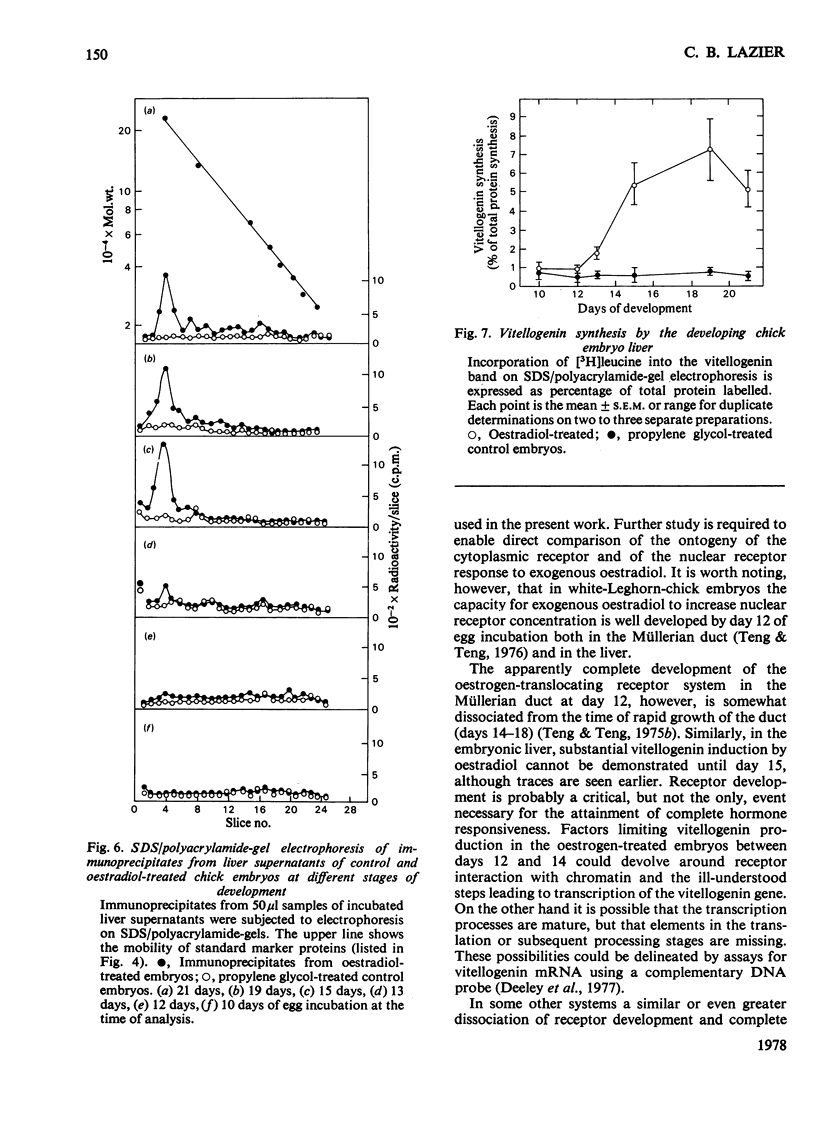
A specific high-affinity oestradiol-binding protein was characterized in salt extracts of liver nuclei of the developing chick embryo. It is present in very small amounts at day 10 of development and is marginally stimulated by oestradiol injection into the yolk sac on day 8. Injection of oestradiol on day 10 evokes a substantial increase in the nuclear oestradiol-binding activity measured on day 12 of development. This oestradiol-binding protein has properties of sedimentation, hormone specificity and high-affinity binding very similar to those of the soluble nuclear receptor in hatched chicks. Livers from the 12-day embryos injected 48 h earlier with oestradiol do not synthesize vitellogenin, as judged by a specific immunochemical and electrophoretic assay for this oestrogen-induced protein. Traces of vitellogenin synthesis can be induced in 13-day-embryo liver, and a substantial response, equivalent to that in hatched chicks, is seen in liver from 15-day embryos injected on day 13. The development of the ability of oestradiol to increase the concentration of the soluble nuclear receptor appears to be one, but not the only, critical factor involved in the development of the ability of chick liver to synthesize vitellogenin.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansari A. Q., Dolphin P. J., Lazier C. B., Munday K. A., Akhtar M. Chemical composition of an oestrogen-induced calcium-binding glycolipophosphoprotein in Xenopus laevis. Biochem J. 1971 Mar;122(1):107–113. doi: 10.1042/bj1220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergink E. W., Kloosterboer H. J., Gruber M., Ab G. Estrogen-induced phosphoprotein synthesis in roosters. Kinetics of induction. Biochim Biophys Acta. 1973 Feb 4;294(1):497–506. doi: 10.1016/0005-2787(73)90105-6. [DOI] [PubMed] [Google Scholar]
- Bergink E. W., Wallace R. A. Precursor-product relationship between amphibian vitellogenin and the yolk proteins, lipovitellin and phosvitin. J Biol Chem. 1974 May 10;249(9):2897–2903. [PubMed] [Google Scholar]
- Carinci P., Caruso A., Evangelisti R., Becchetti E., Stabellini G. Studies on the mechanism of in vitro estradiol-17 beta induced synthesis of phosvitin in chick embryo liver cells. Cell Differ. 1976 Mar;4(6):441–448. doi: 10.1016/0045-6039(76)90030-0. [DOI] [PubMed] [Google Scholar]
- Chan L., Jackson R. L., O'Malley B. W., Means A. R. Synthesis of very low density lipoproteins in the cockerel. Effects of estrogen. J Clin Invest. 1976 Aug;58(2):368–379. doi: 10.1172/JCI108481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens M. J. The regulation of egg yolk protein synthesis by steroid hormones. Prog Biophys Mol Biol. 1974;28:69–108. doi: 10.1016/0079-6107(74)90017-0. [DOI] [PubMed] [Google Scholar]
- Deeley R. G., Gordon J. I., Burns A. T., Mullinix K. P., Binastein M., Goldberg R. F. Primary activation of the vitellogenin gene in the rooster. J Biol Chem. 1977 Nov 25;252(22):8310–8319. [PubMed] [Google Scholar]
- Deeley R. G., Mullinix D. P., Wetekam W., Kronenberg H. M., Meyers M., Eldridge J. D., Goldberger R. F. Vitellogenin synthesis in the avian liver. Vitellogenin is the precursor of the egg yolk phosphoproteins. J Biol Chem. 1975 Dec 10;250(23):9060–9066. [PubMed] [Google Scholar]
- Feldman D. Ontogeny of rat hepatic glucocorticoid receptors. Endocrinology. 1974 Nov;95(5):1219–1227. doi: 10.1210/endo-95-5-1219. [DOI] [PubMed] [Google Scholar]
- Geratz J. D., Cheng M. C., Tidwell R. R. Novel bis(benzamidino) compounds with an aromatic central link. Inhibitors of thrombin, pancreatic kallikrein, trypsin, and complement. J Med Chem. 1976 May;19(5):634–639. doi: 10.1021/jm00227a011. [DOI] [PubMed] [Google Scholar]
- Giannopoulos G. Variations in the levels of cytoplasmic glucocorticoid receptors in lungs of various species at different developmental stages. Endocrinology. 1974 Feb;94(2):450–458. doi: 10.1210/endo-94-2-450. [DOI] [PubMed] [Google Scholar]
- Green C. D., Tata J. R. Direct induction by estradiol on vitellogenin synthesis in organ cultures of male Xenopus laevis liver. Cell. 1976 Jan;7(1):131–139. doi: 10.1016/0092-8674(76)90263-4. [DOI] [PubMed] [Google Scholar]
- Gschwendt M. A cytoplasmic oestrogen-binding component in chicken liver. Hoppe Seylers Z Physiol Chem. 1975 Feb;356(2):157–165. doi: 10.1515/bchm2.1975.356.1.157. [DOI] [PubMed] [Google Scholar]
- Gschwendt M. Estrogen binding sitesin the embryonic chicken liver. FEBS Lett. 1977 Mar 15;75(1):272–276. doi: 10.1016/0014-5793(77)80101-4. [DOI] [PubMed] [Google Scholar]
- Gschwendt M., Kittstein W. A cytoplasmic high affinity estrogen-binding protein in the embryonic chicken liver. Eur J Biochem. 1977 Nov 1;80(2):461–468. doi: 10.1111/j.1432-1033.1977.tb11901.x. [DOI] [PubMed] [Google Scholar]
- HEALD P. J., MCLACHLAN P. M. THE SYNTHESIS OF PHOSVITIN IN VITRO BY SLICES OF LIVER FROM THE LAYING HEN. Biochem J. 1965 Jan;94:32–39. doi: 10.1042/bj0940032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison R. W., Toft D. O. Estrogen receptors in the chick oviduct. Endocrinology. 1975 Jan;96(1):199–205. doi: 10.1210/endo-96-1-199. [DOI] [PubMed] [Google Scholar]
- Jailkhani B. L., Talwar G. P. Induction of phosvitin by oestradiol in rooster liver needs DNA synthesis. Nat New Biol. 1972 Oct 25;239(95):240–241. doi: 10.1038/newbio239240a0. [DOI] [PubMed] [Google Scholar]
- Joss U., Bassand C., Dierks-Ventling C. Rapid appearance of estrogen receptor in chick liver nuclei: partial inhibition by cycloheximide. FEBS Lett. 1976 Jul 15;66(2):293–298. doi: 10.1016/0014-5793(76)80525-x. [DOI] [PubMed] [Google Scholar]
- Jost J. P., Pehling G., Baca O. G. Rate of synthesis of beta L-lipovitellin in the liver of immature chicks treated with 17beta estradiol. Biochem Biophys Res Commun. 1975 Feb 17;62(4):957–965. doi: 10.1016/0006-291x(75)90416-7. [DOI] [PubMed] [Google Scholar]
- Jost J. P., Pehling G. Organization of vitellogenin polysomes, size of the mRNA and polyadenylate fragment. Eur J Biochem. 1976 Feb 16;62(2):299–306. doi: 10.1111/j.1432-1033.1976.tb10161.x. [DOI] [PubMed] [Google Scholar]
- Kato J., Atsumi Y., Inaba M. Estradiol receptors in female rat hypothalamus in the developmental stages and during pubescence. Endocrinology. 1974 Feb;94(2):309–317. doi: 10.1210/endo-94-2-309. [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen B. S., Greger N. G. Ontogeny of uterine responsiveness to estrogen during early development in the rat. Mol Cell Endocrinol. 1974 Dec;2(1):31–42. doi: 10.1016/0303-7207(74)90010-0. [DOI] [PubMed] [Google Scholar]
- Kaye A. M., Icekson I., Lamprecht S. A., Gruss R., Tsafriri A., Lindner H. R. Stimulation of ornithine decarboxylase activity by luteinizing hormone in immature and adult rat ovaries. Biochemistry. 1973 Jul 31;12(16):3072–3076. doi: 10.1021/bi00740a020. [DOI] [PubMed] [Google Scholar]
- Kaye A. M., Sheratzky D., Lindner H. R. Kinetics of DNA synthesis in immature rat uterus: age dependence and estradiol stimulation. Biochim Biophys Acta. 1971 Feb 28;261(2):475–486. doi: 10.1016/0304-4165(72)90072-4. [DOI] [PubMed] [Google Scholar]
- Lazier C. B., Alford W. S. Interaction of the anti-oestrogen, nafoxidine hydrochloride, with the soluble nuclear oestradiol-binding protein in chick liver. Biochem J. 1977 Jun 15;164(3):659–667. doi: 10.1042/bj1640659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazier C. (3H)-estradiol binding by chick liver nuclear extracts: mechanism of increase in binding following estradiol injection. Steroids. 1975 Sep;26(3):281–298. doi: 10.1016/0039-128x(75)90075-6. [DOI] [PubMed] [Google Scholar]
- Lippman M. E., Wiggert B. O., Chader G. J., Thompson E. B. Glucocorticoid receptors. Characteristics, specificity, and ontogenesis in embryonic chick neural retina. J Biol Chem. 1974 Sep 25;249(18):5916–5917. [PubMed] [Google Scholar]
- Luskey K. L., Brown M. S., Goldstein J. L. Stimulation of the synthesis of very low density lipoproteins in rooster liver by estradiol. J Biol Chem. 1974 Sep 25;249(18):5939–5947. [PubMed] [Google Scholar]
- Mester J., Baulieu E. E. Nuclear estrogen receptor of chick liver. Biochim Biophys Acta. 1972 Jan 28;261(1):236–244. doi: 10.1016/0304-4165(72)90334-0. [DOI] [PubMed] [Google Scholar]
- Ozon R., Bellé R. Récepteurs de l'oestradiol-17 dans le foie de poule et de l'amphibien Discoglossus pictus. Biochim Biophys Acta. 1973 Jan 24;297(1):155–163. [PubMed] [Google Scholar]
- Rice R. H., Means G. E. Radioactive labeling of proteins in vitro. J Biol Chem. 1971 Feb 10;246(3):831–832. [PubMed] [Google Scholar]
- SCHJEIDE O. A., BINZ S., RAGAN N. Estrogen-induced serum protein synthesis in the liver of the chicken embryo. Growth. 1960 Dec;24:401–410. [PubMed] [Google Scholar]
- Solomon S., Lee D. K. Binding of glucocorticoids in fetal tissues. J Steroid Biochem. 1977 May;8(5):453–461. doi: 10.1016/0022-4731(77)90247-3. [DOI] [PubMed] [Google Scholar]
- Somjen G. J., Kaye A. M., Linder H. R. Oestradiol-17 beta binding proteins in the rat uterus: changes during postnatal development. Mol Cell Endocrinol. 1974 Oct;1(5):341–353. doi: 10.1016/0303-7207(74)90023-9. [DOI] [PubMed] [Google Scholar]
- Somjen G. J., Kaye A. M., Lindner H. R. Demonstration of 8 S-cytoplasmic oestrogen receptor in rat mullerian duct. Biochim Biophys Acta. 1976 May 28;428(3):787–791. doi: 10.1016/0304-4165(76)90210-5. [DOI] [PubMed] [Google Scholar]
- Sömjen D., Sömjen G., King R. J., Kaye A. M., Lindner H. R. Nuclear binding of oestradiol-17beta and induction of protein synthesis in the rat uterus during postnatal development. Biochem J. 1973 Sep;136(1):25–33. doi: 10.1042/bj1360025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata J. R. The expression of the vitellogenin gene. Cell. 1976 Sep;9(1):1–14. doi: 10.1016/0092-8674(76)90047-7. [DOI] [PubMed] [Google Scholar]
- Teng C. S., Teng C. T. Studies on sex-organ development. Isolation and characterization of an oestrogen receptor from chick Müllerian duct. Biochem J. 1975 Aug;150(2):183–190. doi: 10.1042/bj1500183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng C. S., Teng C. T. Studies on sex-organ development. Ontogeny of cytoplasmic oestrogen receptor in chick Müllerian duct. Biochem J. 1975 Aug;150(2):191–194. doi: 10.1042/bj1500191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng C. S., Teng C. T. Study on sex-organ development. Oestrogen-receptor translocation in the developing chick Müllerian duct. Biochem J. 1976 Jan 15;154(1):1–9. doi: 10.1042/bj1540001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangh L. J., Knowland J. Synthesis of vitellogenin in cultures of male and female frog liver regulated by estradiol treatment in vitro. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3172–3175. doi: 10.1073/pnas.72.8.3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]


