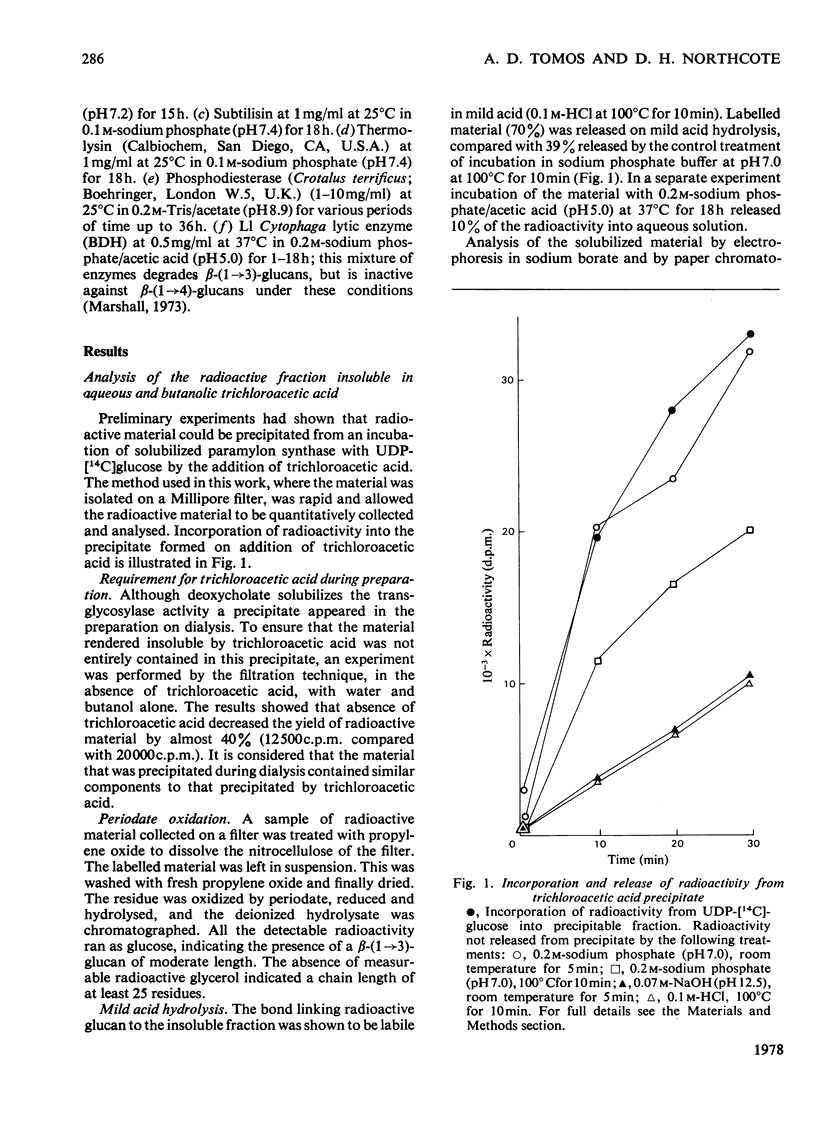
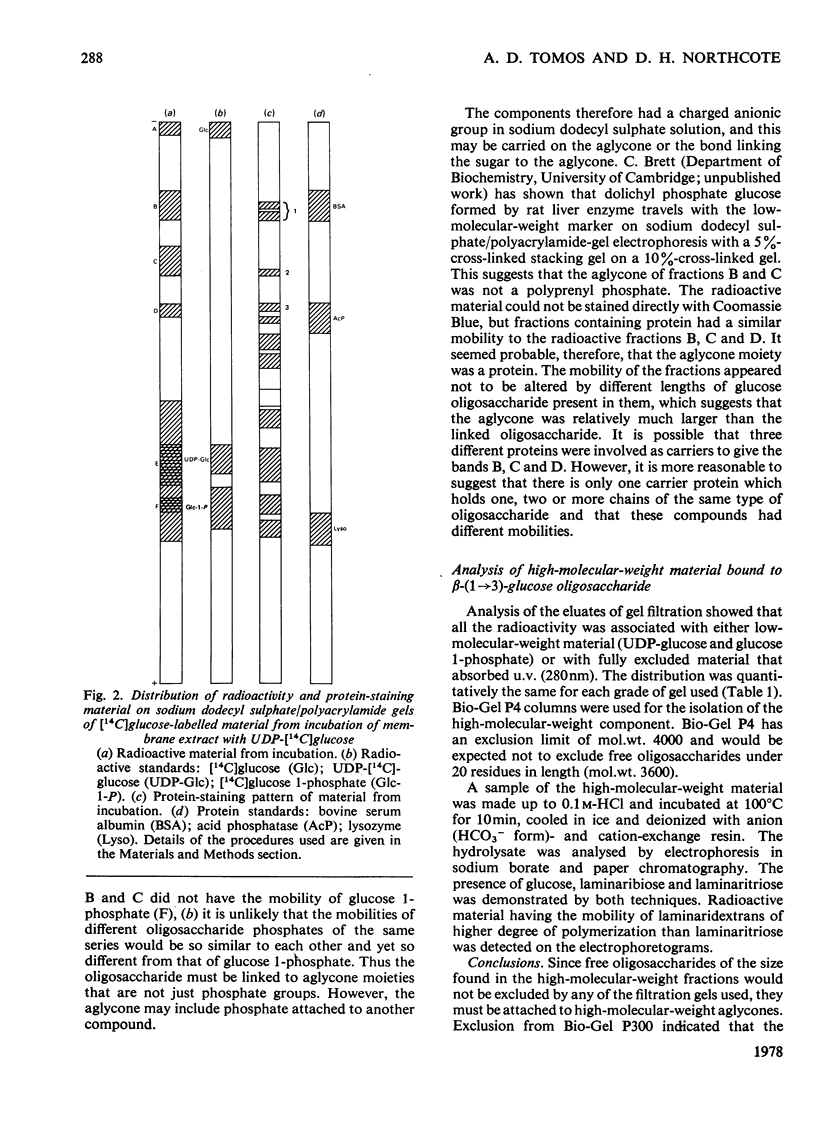
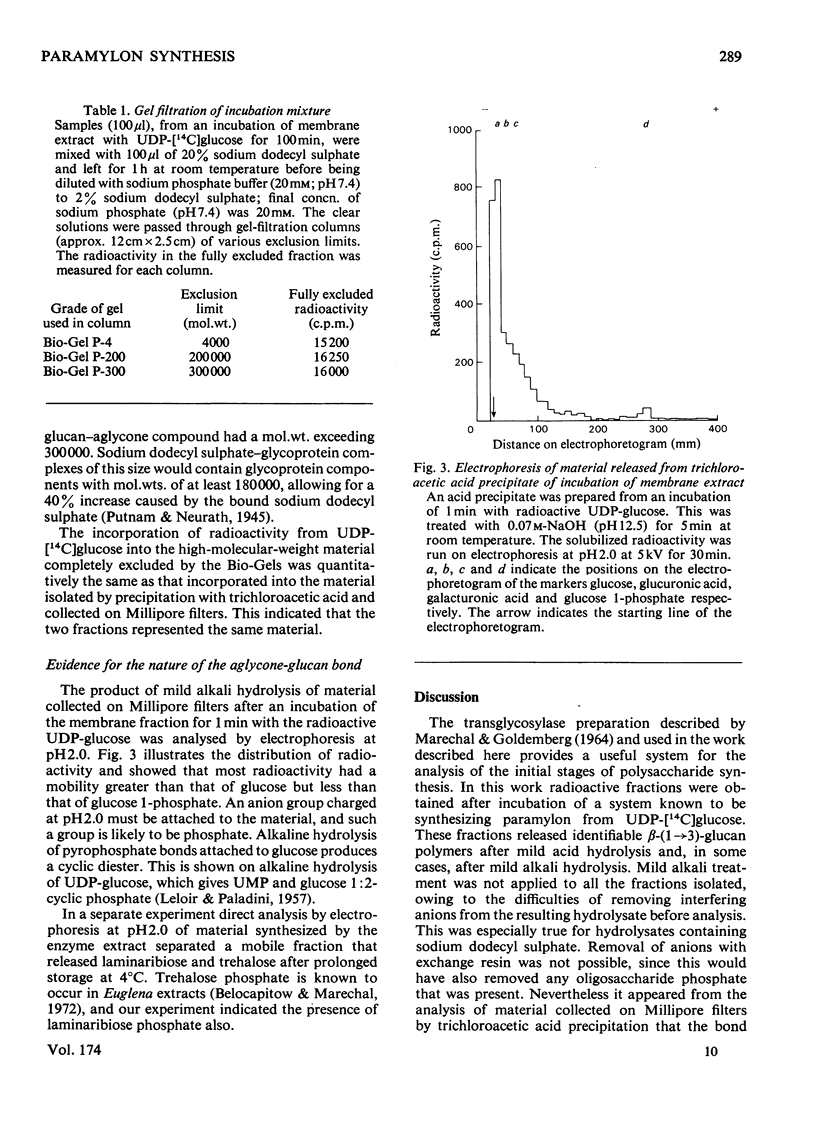
Abstract
A sodium deoxycholate extract containing glucosyltransferase activity was obtained from a particulate preparation from Euglena gracilis. It transferred glucose from UDP-[14C]glucose into material that was precipitated by trichloroacetic acid. This material released beta-(1 leads to 3)-glucan oligosaccharides into solution on incubation with weak acid, weak alkali and beta-(1 leads to 3)-glucosidase. The products of the incubation of the deoxycholate extract with UDP-[14C]glucose were analysed by sodium dodecyl sulphate/polyacrylamide-gel electrophoresis. Radioactive bands were obtained that had the properties of beta-(1 leads to 3)-glucan covalently linked to protein by a bond labile to weak acid. High-molecular-weight material containing a beta-(1 leads to 3)-glucan was also shown to be present by gel filtration. The bond linking glucan to aglycone is possibly a pyrophosphate linkage. It is proposed that in Euglena gracilis beta-(1 leads to 3)-glucan (paramylon) is synthesized on a protein primer.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANDURSKI R. S., AXELROD B. The chromatographic identification of some biologically important phosphate esters. J Biol Chem. 1951 Nov;193(1):405–410. [PubMed] [Google Scholar]
- Barengo R., Flawia M., Krisman C. R. The initiation of glycogen biosynthesis in Escherichia coli. FEBS Lett. 1975 May 15;53(3):274–278. doi: 10.1016/0014-5793(75)80035-4. [DOI] [PubMed] [Google Scholar]
- Berthillier G., Azzar G. J., Got R. Etude de l'activité de transfert de glucose, à partir d'UDP-glucose, dans les membranes microsomiques des hépatocytes de rat. Eur J Biochem. 1975 Feb 3;51(1):275–282. doi: 10.1111/j.1432-1033.1975.tb03927.x. [DOI] [PubMed] [Google Scholar]
- Clark A. F., Villemez C. L. The Formation of beta, 1 --> 4 Glucan from UDP-alpha-d-Glucose Catalyzed by a Phaseolus aureus Enzyme. Plant Physiol. 1972 Sep;50(3):371–374. doi: 10.1104/pp.50.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbein A. D. Biosynthesis of a cell wall glucomannan in mung bean seedlings. J Biol Chem. 1969 Mar 25;244(6):1608–1616. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Gahan L. C., Conrad H. E. An enzyme system for de novo biosynthesis of glycogen in Aerobacter aerogenes. Biochemistry. 1968 Nov;7(11):3979–3990. doi: 10.1021/bi00851a027. [DOI] [PubMed] [Google Scholar]
- Green J. R., Northcote D. H. The structure and function of glycoproteins synthesized during slime-polysaccharide production by membranes of the root-cap cells of maize (Zea mays). Biochem J. 1978 Mar 15;170(3):599–608. doi: 10.1042/bj1700599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P. J., Northcote D. H. Patterns of polysaccharide biosynthesis in differentiating cells of maize root-tips. Biochem J. 1970 Dec;120(3):479–491. doi: 10.1042/bj1200479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krisman C. R. A possible intermediate in the initiation of glycogen biosynthesis. Ann N Y Acad Sci. 1973 Feb 9;210:81–89. doi: 10.1111/j.1749-6632.1973.tb47563.x. [DOI] [PubMed] [Google Scholar]
- Krisman C. R. A possible intermediate in the initiation of glycogen biosynthesis. Biochem Biophys Res Commun. 1972 Feb 16;46(3):1206–1212. doi: 10.1016/s0006-291x(72)80103-7. [DOI] [PubMed] [Google Scholar]
- Krisman C. R., Barengo R. A precursor of glycogen biosynthesis: alpha-1,4-glucan-protein. Eur J Biochem. 1975 Mar 3;52(1):117–123. doi: 10.1111/j.1432-1033.1975.tb03979.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lavintman N., Cardini C. E. Particulate UDP-glucose: Protein transglucosylase from potato tuber. FEBS Lett. 1973 Jan 1;29(1):43–46. doi: 10.1016/0014-5793(73)80011-0. [DOI] [PubMed] [Google Scholar]
- Lavintman N., Tandecarz J., Carceller M., Mendiara S., Cardini C. E. Role of uridine diphosphate glucose in the biosynthesis of starch. Mechanism of formation and enlargement of a glucoproteic acceptor. Eur J Biochem. 1974 Dec 16;50(1):145–155. doi: 10.1111/j.1432-1033.1974.tb03882.x. [DOI] [PubMed] [Google Scholar]
- MARECHAL L. R., GOLDEMBERG S. H. URIDINE DIPHOSPHATE GLUCOSE-BETA-1,3-GLUCAN BETA-3-GLUCOSYLTRANSFERASE FROM EUGLENA GRACILIS. J Biol Chem. 1964 Oct;239:3163–3167. [PubMed] [Google Scholar]
- OLAITAN S. A., NORTHCOTE D. H. Polysaccharides of Chlorella pyrenoidosa. Biochem J. 1962 Mar;82:509–519. doi: 10.1042/bj0820509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Tandecarz J., Lavintman N., Cardini C. E. Biosynthesis of starch. Formation of a glucoproteic acceptor by a potato non-sedimentable preparation. Biochim Biophys Acta. 1975 Aug 13;399(2):345–355. [PubMed] [Google Scholar]


