Abstract
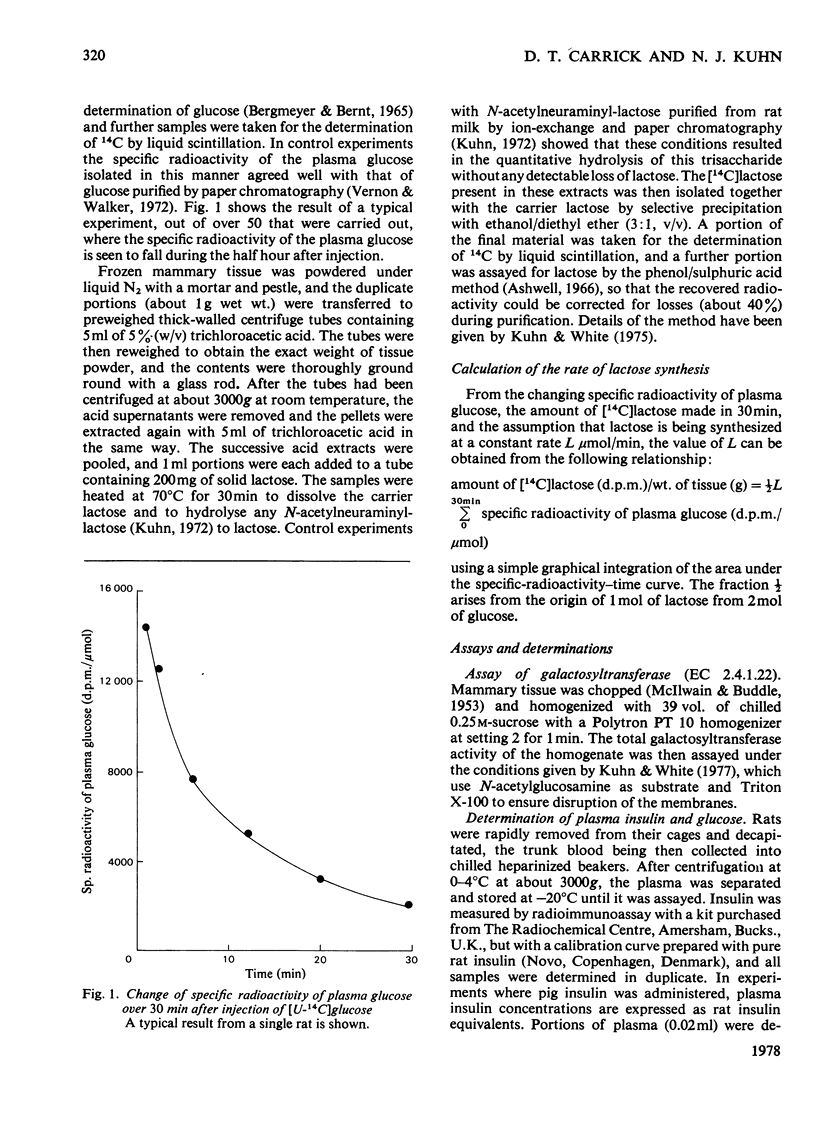
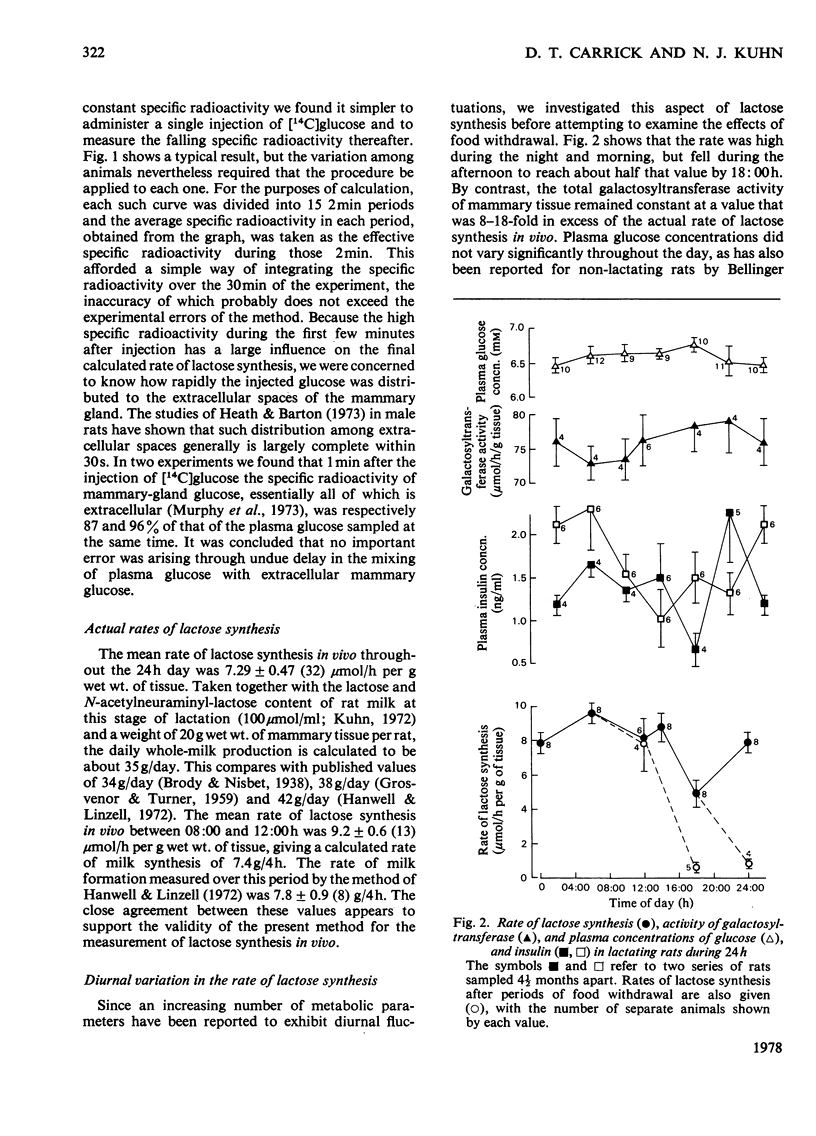
1. The incorporation of radiolabelled plasma glucose into mammary lactose was used to measure the rate of lactose synthesis in lightly anaesthetized lactating rats. 2. Lactose synthesis showed a diurnal variation with a minimum at 18:00h 3. Food withdrawal for 6h did not affect lactose synthesis in the early morning but greatly decreased it in the afternoon or evening. 4. Plasma glucose, milk sugars and total galactosyltransferase activity (EC 2.4.1.22) did not show the above changes. 5. Measurements of plasma insulin, which varies diurnally, and experiments with injected insulin suggested that variations of insulin within the physiological range do not account for the changes in lactose synthesis described.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAHAM S., FITCH W. M., CHAIKOFF I. L. Mannose metabolism and the demonstration of mannokinase and phosphomannoisomerase activities in the lactating rat mammary gland. Arch Biochem Biophys. 1961 May;93:278–282. doi: 10.1016/0003-9861(61)90262-4. [DOI] [PubMed] [Google Scholar]
- BAAR S., BULL J. P. Salt interference in sugar chromatography of urine. Nature. 1953 Aug 29;172(4374):414–415. doi: 10.1038/172414a0. [DOI] [PubMed] [Google Scholar]
- Baird G. D. Fructose-1,6-diphosphatase and phosphopyruvate carboxykinase in bovine lactating mammary gland. Biochim Biophys Acta. 1969 Apr 1;177(2):343–345. doi: 10.1016/0304-4165(69)90145-7. [DOI] [PubMed] [Google Scholar]
- Barra H. S., Cumar F. A., Caputto R. The synthesis of neuramin lactose by preparations of rat mammary gland and its relation to the synthesis of lactose. J Biol Chem. 1969 Nov 25;244(22):6233–6240. [PubMed] [Google Scholar]
- Bartley J. C., Abraham S., Chaikoff I. L. Biosynthesis of lactose by mammary gland slices from the lactating rat. J Biol Chem. 1966 Mar 10;241(5):1132–1137. [PubMed] [Google Scholar]
- Bruckdorfer K. R., Kang S. S., Khan I. H., Bourne A. R., Yudkin J. Diurnal changes in the concentrations of plasma lipids, sugars, insulin and corticosterone in rats fed diets containing various carbohydrates. Horm Metab Res. 1974 Mar;6(2):99–106. doi: 10.1055/s-0028-1093890. [DOI] [PubMed] [Google Scholar]
- Carlson D. M., Jourdian G. W., Roseman S. The sialic acids. XIV. Synthesis of sialyl-lactose by a sialyltransferase from rat mammary gland. J Biol Chem. 1973 Aug 25;248(16):5742–5750. [PubMed] [Google Scholar]
- Carrick D. T., Kuhn N. J. A method for measuring rates of lactose synthesis in vivo in laboratory animals [proceedings]. Biochem Soc Trans. 1977;5(4):1038–1039. doi: 10.1042/bst0051038. [DOI] [PubMed] [Google Scholar]
- GROSVENOR C. E., TURNER C. W. Lactogenic hormone requirements for milk secretion in intact lactating rats. Proc Soc Exp Biol Med. 1959 Aug-Sep;101:699–703. doi: 10.3181/00379727-101-25066. [DOI] [PubMed] [Google Scholar]
- Hanwell A., Linzell J. L. A simple technique for measuring the rate of milk secretion in the rat. Comp Biochem Physiol A Comp Physiol. 1972 Oct 1;43(2):259–270. doi: 10.1016/0300-9629(72)90184-3. [DOI] [PubMed] [Google Scholar]
- Hanwell A., Linzell J. L. The time course of cardiovascular changes in lactation in the rat. J Physiol. 1973 Aug;233(1):93–109. doi: 10.1113/jphysiol.1973.sp010299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath D. F., Barton R. N. The design of experiments using isotopes for the determination of the rates of disposal of blood-borne substrates in vivo with special reference to glucose, ketone bodies, free fatty acids and proteins. Biochem J. 1973 Nov;136(3):503–518. doi: 10.1042/bj1360503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R. L., Brew K. Lactose synthetase. Adv Enzymol Relat Areas Mol Biol. 1975;43:411–490. doi: 10.1002/9780470122884.ch5. [DOI] [PubMed] [Google Scholar]
- Katz J., Dunn A., Chenoweth M., Golden S. Determination of synthesis, recycling and body mass of glucose in rats and rabbits in vivo 3H-and 14C-labelled glucose. Biochem J. 1974 Jul;142(1):171–183. doi: 10.1042/bj1420171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T., Maji T., Ashida K. Periodicity of food intake and lipogenesis in rats subjected to two different feeding plans. J Nutr. 1970 Jun;100(6):691–697. doi: 10.1093/jn/100.6.691. [DOI] [PubMed] [Google Scholar]
- Kuhn N. J. Lactogenesis in the rat. Metabolism of uridine diphosphate galactose by mammary gland. Biochem J. 1968 Feb;106(3):743–748. doi: 10.1042/bj1060743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn N. J. The lactose and neuraminlactose content of rat milk and mammary tissue. Biochem J. 1972 Nov;130(1):177–180. doi: 10.1042/bj1300177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn N. J., White A. The role of nucleoside diphosphatase in a uridine nucleotide cycle associated with lactose synthesis in rat mammary-gland Golgi apparatus. Biochem J. 1977 Dec 15;168(3):423–433. doi: 10.1042/bj1680423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn N. J., White A. The topography of lactose synthesis. Biochem J. 1975 Apr;148(1):77–84. doi: 10.1042/bj1480077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzell J. L. The effect of infusions of glucose, acetate and amino acids on hourly milk yield in fed, fasted and insulin-treated goats. J Physiol. 1967 May;190(2):347–357. doi: 10.1113/jphysiol.1967.sp008213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCILWAIN H., BUDDLE H. L. Techniques in tissue metabolism. I. A mechanical chopper. Biochem J. 1953 Feb;53(3):412–420. doi: 10.1042/bj0530412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. J., Baldwin R. L. Effects of insulin on isolated rat mammary cell metabolism: glucose utilization and metabolite patterns. Endocrinology. 1971 Nov;89(5):1263–1269. doi: 10.1210/endo-89-5-1263. [DOI] [PubMed] [Google Scholar]
- Murphy G., Ariyanayagam A. D., Kuhn N. J. Progesterone and the metabolic control of the lactose biosynthetic pathway during lactogenesis in the rat. Biochem J. 1973 Dec;136(4):1105–1116. doi: 10.1042/bj1361105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J. T., Brew K. Metal ion activation of galactosyltransferase. J Biol Chem. 1976 Jun 25;251(12):3645–3652. [PubMed] [Google Scholar]
- Robinson A. M., Williamson D. H. Comparison of glucose metabolism in the lactating mammary gland of the rat in vivo and in vitro. Effects of starvation, prolactin or insulin deficiency. Biochem J. 1977 Apr 15;164(1):153–159. doi: 10.1042/bj1640153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A. M., Williamson D. H. Effects of acetoacetate administration on glucose metabolism in mammary gland of fed lactating rats. Biochem J. 1977 Jun 15;164(3):749–752. doi: 10.1042/bj1640749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon R. G., Walker D. G. Glucose metabolism in the developing rat. Studies in vivo. Biochem J. 1972 Apr;127(3):521–529. doi: 10.1042/bj1270521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde C. J., Kuhn N. J. Lactose synthesis, galactosyltransferase activity and plasma insulin concentration throughout lactation in the rat [proceedings]. Biochem Soc Trans. 1977;5(4):1040–1041. doi: 10.1042/bst0051040. [DOI] [PubMed] [Google Scholar]


