Abstract
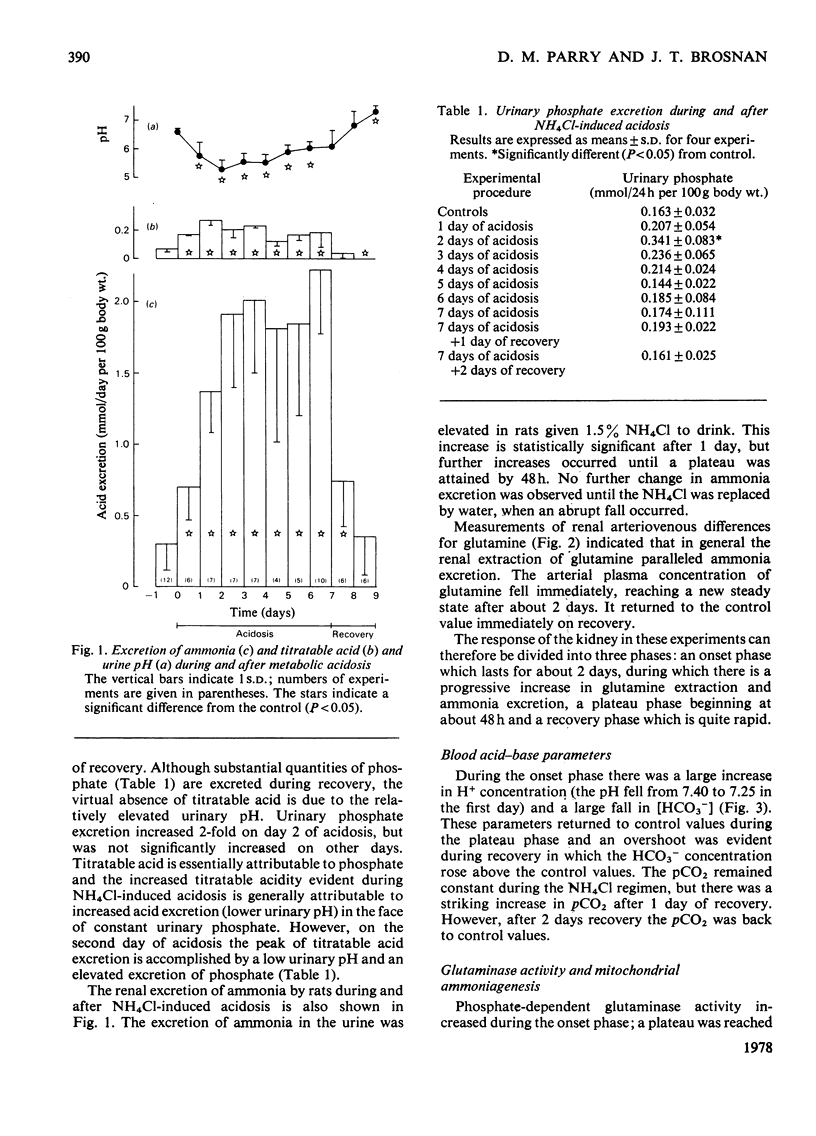
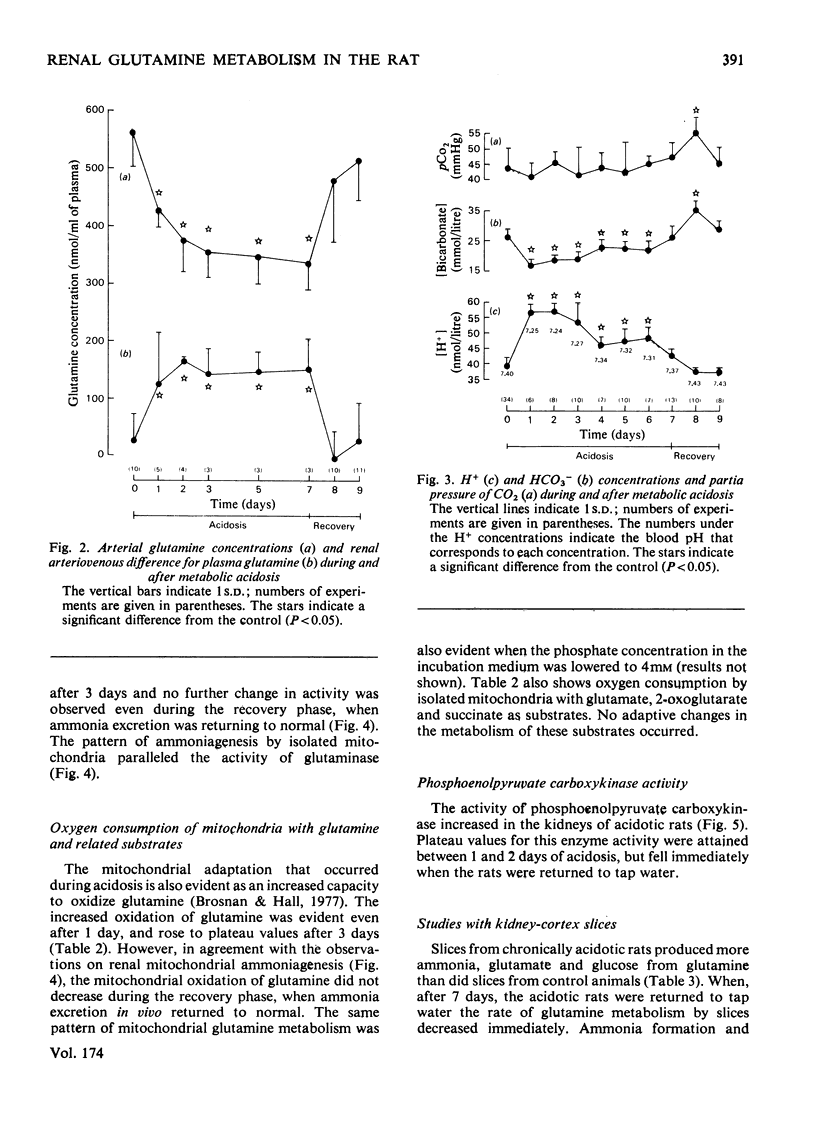
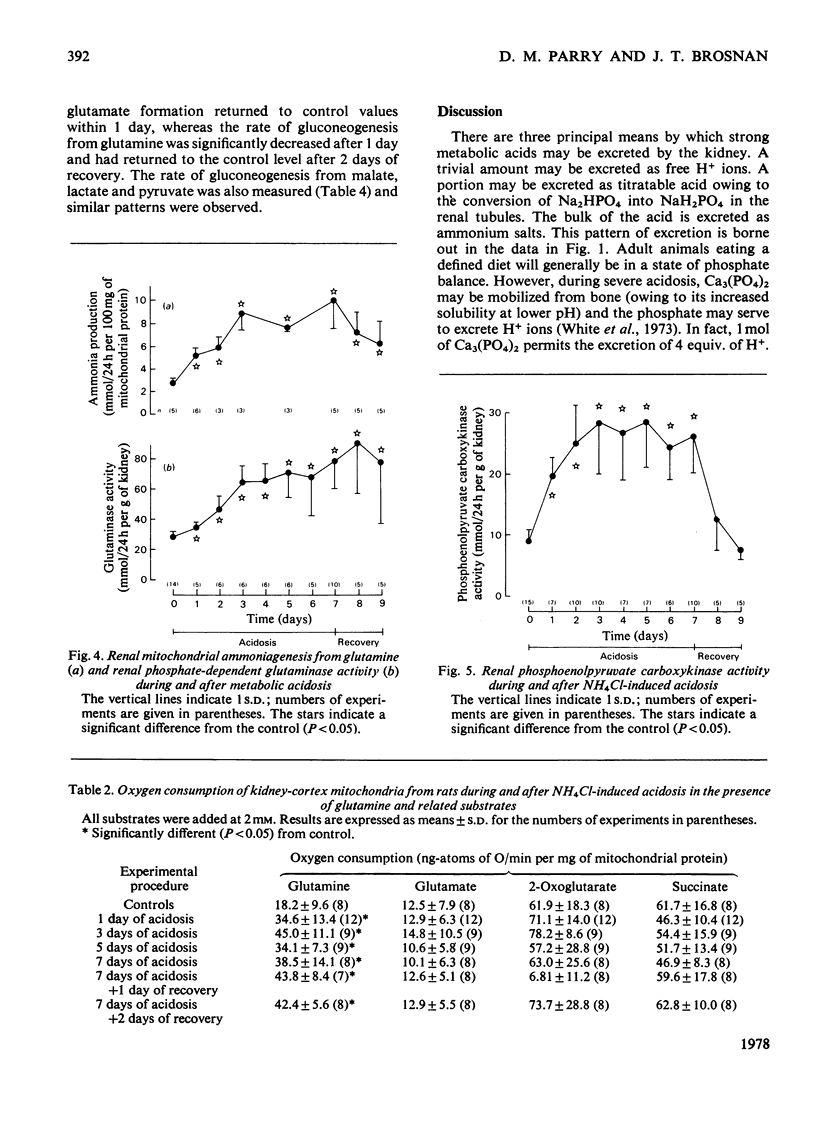
Experiments were carried out on rats to evaluate the possible regulatory roles of renal glutaminase activity, mitochondrial permeability to glutamine, phosphoenolpyruvate carboxykinase activity and systemic acid–base changes in the control of renal ammonia (NH3 plus NH4+) production. Acidosis was induced by drinking NH4Cl solution ad libitum. A pronounced metabolic acidosis without respiratory compensation [pH=7.25; HCO3−=16.9mequiv./litre; pCO2=40.7mmHg (5.41kPa)] was evident for the first 2 days, but thereafter acid–base status returned towards normal. This improvement in acid–base status was accompanied by the attainment of maximal rates of ammonia excretion (onset phase) after about 2 days. A steady rate of ammonia excretion was then maintained (plateau phase) until the rats were supplied with tap water in place of the NH4Cl solution, whereupon pCO2 and HCO3− became elevated [55.4mmHg (7.37kPa) and 35.5mequiv./litre] and renal ammonia excretion returned to control values within 1 day (recovery phase). Renal arteriovenous differences for glutamine always paralleled rates of ammonia excretion. Phosphate-dependent glutaminase and phosphoenolpyruvate carboxykinase activities and the rate of glutamine metabolism (NH3 production and O2 consumption) by isolated kidney mitochondria all increased during the onset phase. The increases in glutaminase and in mitochondrial metabolism continued into the plateau phase, whereas the increase in the carboxykinase reached a plateau at the same time as did ammonia excretion. During the recovery phase a rapid decrease in carboxykinase activity accompanied the decrease in ammonia excretion, whereas glutaminase and mitochondrial glutamine metabolism in vitro remained elevated. The metabolism of glutamine by kidney-cortex slices (ammonia, glutamate and glucose production) paralleled the metabolism of glutamine in vivo during recovery, i.e. it returned to control values. The results indicate that the adaptations in mitochondrial glutamine metabolism must be regulated by extra-mitochondrial factors, since glutamine metabolism in vivo and in slices returns to control values during recovery, whereas the mitochondrial metabolism of glutamine remains elevated.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam W., Simpson D. P. Glutamine transport in rat kidney mitochondria in metabolic acidosis. J Clin Invest. 1974 Jul;54(1):165–174. doi: 10.1172/JCI107738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alleyne G. A. Renal metabolic response to acid-base changes. II. The early effects of metabolic acidosis on renal metabolism in the rat. J Clin Invest. 1970 May;49(5):943–951. doi: 10.1172/JCI106314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alleyne G. A., Scullard G. H. Renal metabolic response to acid base changes. I. Enzymatic control of ammoniagenesis in the rat. J Clin Invest. 1969 Feb;48(2):364–370. doi: 10.1172/JCI105993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett F. I., Alleyne G. A. Gluconeogenesis and ammoniagenesis in rat kidney: effect of 3-mercaptopicolinic acid. FEBS Lett. 1976 Jun 1;65(2):215–219. doi: 10.1016/0014-5793(76)80483-8. [DOI] [PubMed] [Google Scholar]
- Benyajati S., Goldstein L. Renal glutaminase adaptation and ammonia excretion in infant rats. Am J Physiol. 1975 Mar;228(3):693–698. doi: 10.1152/ajplegacy.1975.228.3.693. [DOI] [PubMed] [Google Scholar]
- Bignall M. C., Elebute O., Lotspeich W. D. Renal protein and ammonia biochemistry in NH4Cl acidosis and after uninephrectomy. Am J Physiol. 1968 Aug;215(2):289–295. doi: 10.1152/ajplegacy.1968.215.2.289. [DOI] [PubMed] [Google Scholar]
- Brosnan J. T., Hall B. The transport and metabolism of glutamine by kidney-cortex mitochondria from normal and acidotic rats. Biochem J. 1977 May 15;164(2):331–337. doi: 10.1042/bj1640331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill G. F., Jr, Aoki T. T. Renal gluconeogenesis and amino-acid metabolism in man. Med Clin North Am. 1975 May;59(3):751–761. doi: 10.1016/s0025-7125(16)32021-1. [DOI] [PubMed] [Google Scholar]
- Curthoys N. P., Lowry O. H. The distribution of glutaminase isoenzymes in the various structures of the nephron in normal, acidotic, and alkalotic rat kidney. J Biol Chem. 1973 Jan 10;248(1):162–168. [PubMed] [Google Scholar]
- Dies F., Lotspeich W. D. Hexose monophosphate shunt in the kidney during acid-base and electrolyte imbalance. Am J Physiol. 1967 Jan;212(1):61–71. doi: 10.1152/ajplegacy.1967.212.1.61. [DOI] [PubMed] [Google Scholar]
- Goldstein L. Glutamine transport by mitochondria isolated from normal and acidotic rats. Am J Physiol. 1975 Oct;229(4):1027–1033. doi: 10.1152/ajplegacy.1975.229.4.1027. [DOI] [PubMed] [Google Scholar]
- Goodman A. D., Fuisz R. E., Cahill G. F., Jr Renal gluconeogenesis in acidosis, alkalosis, and potassium deficiency: its possible role in regulation of renal ammonia production. J Clin Invest. 1966 Apr;45(4):612–619. doi: 10.1172/JCI105375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guder W. G., Schmidt U. The localization of gluconeogenesis in rat nephron. Determination of phosphoenolpyruvate carboxykinase in microdissected tubules. Hoppe Seylers Z Physiol Chem. 1974 Mar;355(3):273–278. doi: 10.1515/bchm2.1974.355.1.273. [DOI] [PubMed] [Google Scholar]
- Hird F. J., Marginson M. A. The formation of ammonia from glutamine and glutamate by mitochondria from rat liver and kidney. Arch Biochem Biophys. 1968 Sep 20;127(1):718–724. doi: 10.1016/0003-9861(68)90282-8. [DOI] [PubMed] [Google Scholar]
- Iynedjian P. B., Ballard F. J., Hanson R. W. The regulation of phosphoenolpyruvate carboxykinase (GTP) synthesis in rat kidney cortex. The role of acid-base balance and glucocorticoids. J Biol Chem. 1975 Jul 25;250(14):5596–5603. [PubMed] [Google Scholar]
- KIRSTEN E., GEREZ C., KIRSTEN R. [An enzymatic microdetermination method for ammonia, specifically for extracts of animal tissues and fluids. Determination of NH4 ions in blood]. Biochem Z. 1963;337:312–319. [PubMed] [Google Scholar]
- KREBS H. A., BENNETT D. A., DE GASQUET P., GASQUET P., GASCOYNE T., YOSHIDA T. Renal gluconeogenesis. The effect of diet on the gluconeogenic capacity of rat-kidney-cortex slices. Biochem J. 1963 Jan;86:22–27. doi: 10.1042/bj0860022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra J., Brosnan J. T. The subcellular localization of glutaminase isoenzymes in rat kidney cortex. J Biol Chem. 1974 May 25;249(10):3255–3260. [PubMed] [Google Scholar]
- Levine D. Z., Nash L. A. Effect of chronic NH4Cl acidosis on proximal tubular H2O and HCO3 reabsorption. Am J Physiol. 1973 Aug;225(2):380–384. doi: 10.1152/ajplegacy.1973.225.2.380. [DOI] [PubMed] [Google Scholar]
- Longshaw I. D., Alleyne G. A., Pogson C. I. The effect of steroids and ammonium chloride acidosis on phosphoenolpyruvate carboxykinase in rat kidney cortex. II. The kinetics of enzyme induction. J Clin Invest. 1972 Sep;51(9):2284–2291. doi: 10.1172/JCI107038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NUTBOURNE D. M. The effect of dilution on the titratable acid in urine and acidified phosphate buffer solutions, and the correction for this effect in the determination of the rate of elimination of hydrogen ions from the body by the renal tubules. Clin Sci. 1961 Apr;20:263–278. [PubMed] [Google Scholar]
- OWEN E. E., ROBINSON R. R. Amino acid extraction and ammonia metabolism by the human kidney during the prolonged administration of ammonium chloride. J Clin Invest. 1963 Feb;42:263–276. doi: 10.1172/JCI104713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts R. F. Symposium on acid-base homeostasis. Control of renal production of ammonia. Kidney Int. 1972 May;1(5):297–305. doi: 10.1038/ki.1972.42. [DOI] [PubMed] [Google Scholar]
- Pitts R. F. The role of ammonia production and excretion in regulation of acid-base balance. N Engl J Med. 1971 Jan 7;284(1):32–38. doi: 10.1056/NEJM197101072840110. [DOI] [PubMed] [Google Scholar]
- RECTOR F. C., Jr, ORLOFF J. The effect of the administration of sodium bicarbonate and ammonium chloride on the excretion and production of ammonia; the absence of alterations in the activity of renal ammonia-producing enzymes in the dog. J Clin Invest. 1959 Feb;38(2):366–372. doi: 10.1172/JCI103810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RECTOR F. C., Jr, SELDIN D. W., COPENHAVER J. H. The mechanism of ammonia excretion during ammonium chloride acidosis. J Clin Invest. 1955 Jan;34(1):20–26. doi: 10.1172/JCI103058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognstad R., Katz J. Gluconeogenesis in the kidney cortex. Effects of D-malate and amino-oxyacetate. Biochem J. 1970 Feb;116(3):483–491. doi: 10.1042/bj1160483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross B. D. Effect of inhibition of gluconeogenesis on ammonia production in the perfused rat kidney. Clin Sci Mol Med. 1976 Jun;50(6):493–498. doi: 10.1042/cs0500493. [DOI] [PubMed] [Google Scholar]
- Schmidt U., Guder W. G. Sites of enzyme activity along the nephron. Kidney Int. 1976 Mar;9(3):233–242. doi: 10.1038/ki.1976.26. [DOI] [PubMed] [Google Scholar]
- Seubert W., Huth W. On the mechanism of gluconeogenesis and its regulation. II. The mechanism of gluconeogenesis from pyruvate and fumarate. Biochem Z. 1965 Nov 15;343(2):176–191. [PubMed] [Google Scholar]
- Sherrard D. J., Simpson D. P. An improved method for the microdetermination of glutamine in plasma and urine. J Lab Clin Med. 1969 May;73(5):877–882. [PubMed] [Google Scholar]
- Simpson D. P., Adam W. Glutamine transport and metabolism by mitochondria from dog renal cortex. General properties and response to acidosis and alkalosis. J Biol Chem. 1975 Oct 25;250(20):8148–8158. [PubMed] [Google Scholar]
- Squires E. J., Hall D. E., Brosnan J. T. Arteriovenous differences for amino acids and lactate across kidneys of normal and acidotic rats. Biochem J. 1976 Oct 15;160(1):125–128. doi: 10.1042/bj1600125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welbourne T., Weber M., Bank N. The effect of glutamine administration on urinary ammonium excretion in normal subjects and patients with renal disease. J Clin Invest. 1972 Jul;51(7):1852–1860. doi: 10.1172/JCI106987. [DOI] [PMC free article] [PubMed] [Google Scholar]


