Abstract
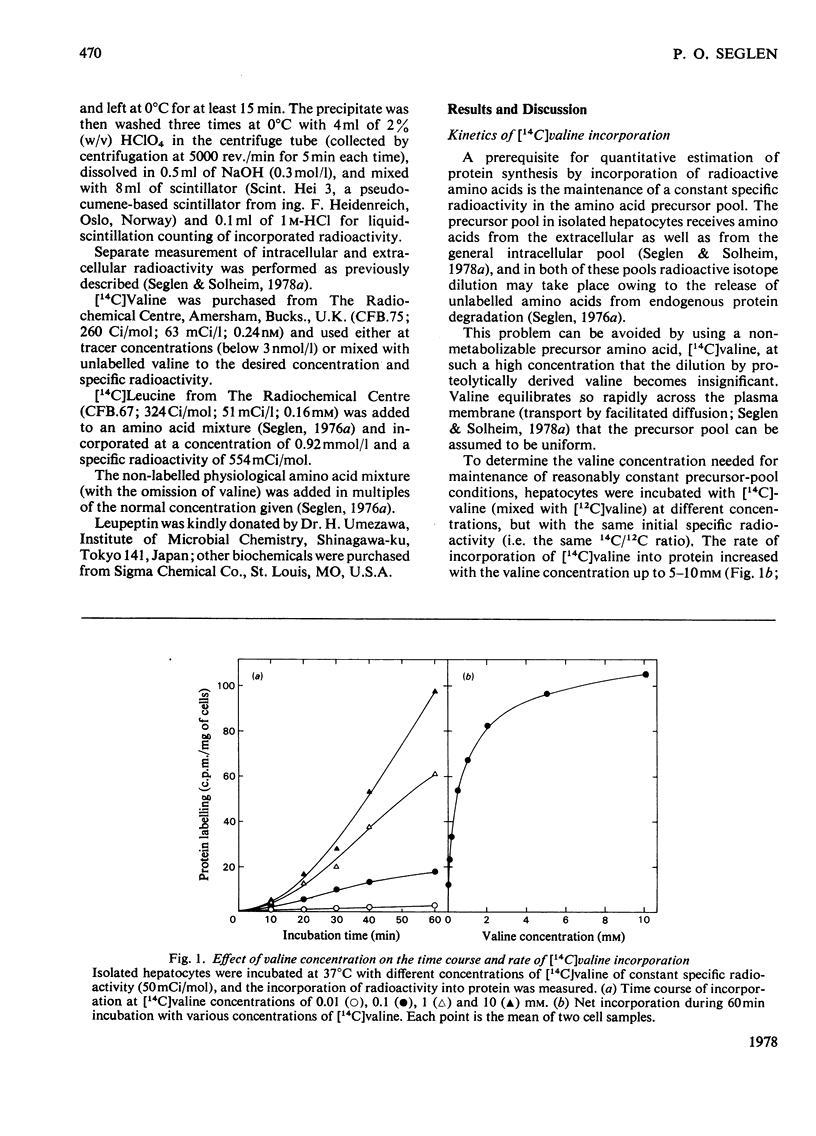
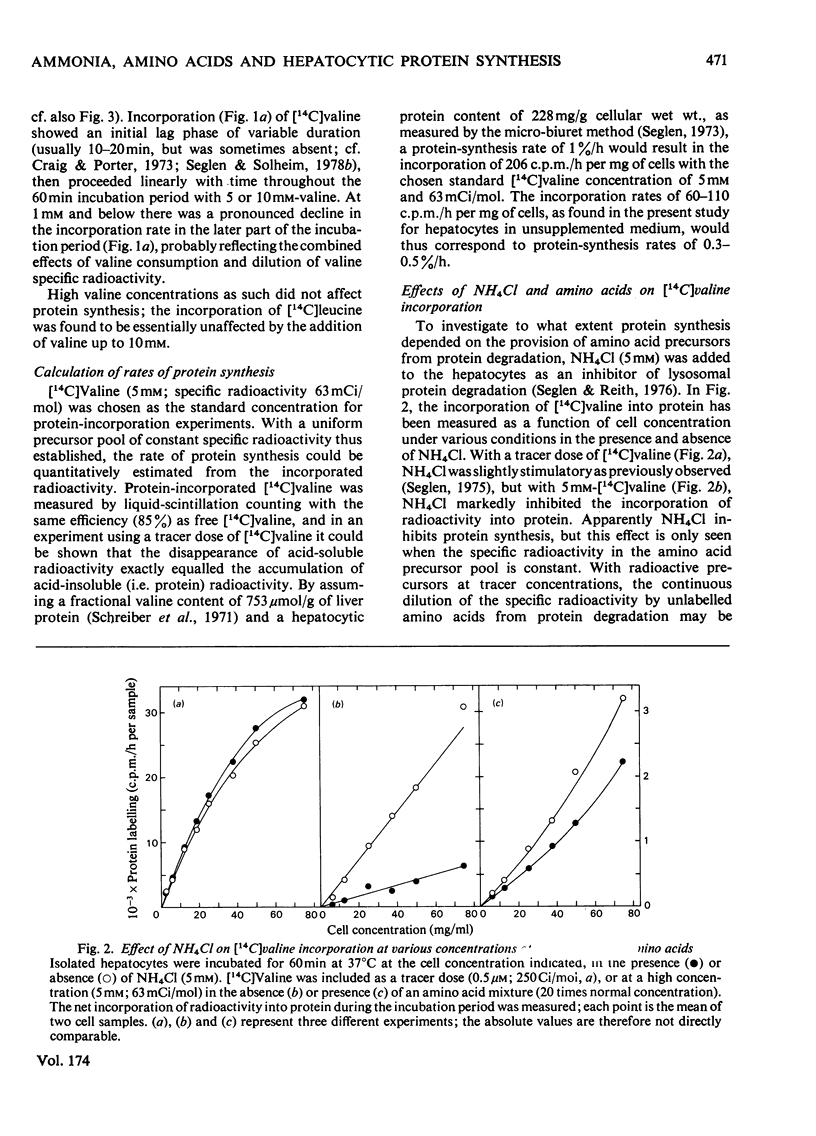
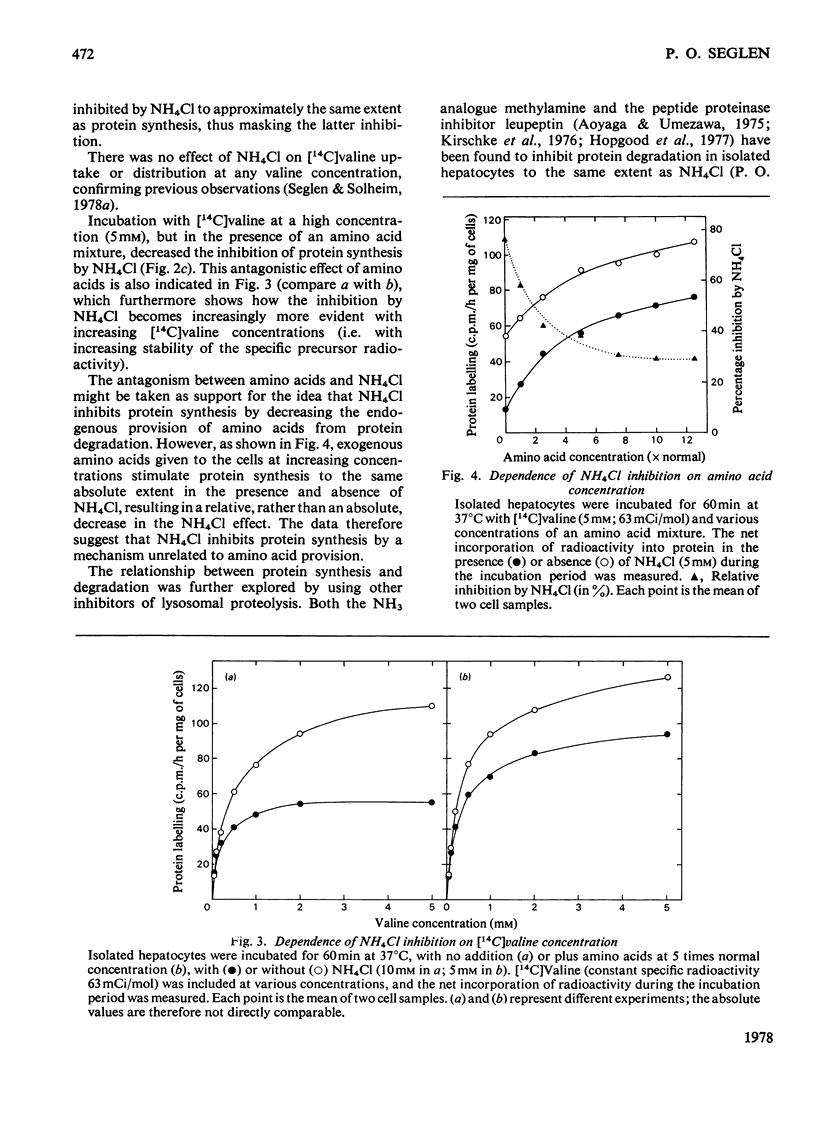
Protein synthesis in isolated rat hepatocytes, as measured by the incorporation of [14C]-valine at constant specific radioactivity, proceeded at a rate of 0.3-0.5%/h in an unsupplemented medium, i.e. only about one-tenth the rate of protein degradation (4%/h). Leupeptin, which inhibits lysosomal protein degradation (previously found to be 75% of the total degradation in hepatocytes), had no effect on protein synthesis, showing that endogenous protein degradation supplied amino acids in excess of the substrate requirements for protein synthesis. The inhibition of protein synthesis by NH4Cl (another inhibitor of lysosomal protein degradation) as well as the stimulation by a physiological amino acid mixture must therefore represent indirect effects, either on general energy metabolism, or on unknown regulatory processes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ekren T., Jervell K. F., Seglen P. O. Insulin and amino-acid regulation of polysomes in perfused, diabetic rat liver. Nat New Biol. 1971 Feb 24;229(8):244–245. doi: 10.1038/newbio229244a0. [DOI] [PubMed] [Google Scholar]
- Eliasson E., Bauer G. E., Hultin T. Reversible degradation of polyribosomes in Chang cells cultured in a glutamine-deficient medium. J Cell Biol. 1967 May;33(2):287–297. doi: 10.1083/jcb.33.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausto N. RNA metabolism in isolated perfused normal and regenerating livers: polyamine effects. Biochim Biophys Acta. 1972 Nov 9;281(4):543–553. doi: 10.1016/0005-2787(72)90155-4. [DOI] [PubMed] [Google Scholar]
- Gan J. C., Jeffay H. The kinetics of transfer of plasma amino acids to tissues, and the turnover rates of liver and muscle proteins. Biochim Biophys Acta. 1971 Oct;252(1):125–135. doi: 10.1016/0304-4165(71)90100-0. [DOI] [PubMed] [Google Scholar]
- Grant A. G., Black E. G. Polyribosome aggregation in isolated rat-liver cells. A comparison of extraction and incubation techniques. Eur J Biochem. 1974 Sep 1;47(2):397–401. doi: 10.1111/j.1432-1033.1974.tb03705.x. [DOI] [PubMed] [Google Scholar]
- Hod Y., Hershko A. Relationship of the pool of intracellular valine to protein synthesis and degradation in cultured cells. J Biol Chem. 1976 Jul 25;251(14):4458–4457. [PubMed] [Google Scholar]
- Hopgood M. F., Clark M. G., Ballard F. J. Inhibition of protein degradation in isolated rat hepatocytes. Biochem J. 1977 May 15;164(2):399–407. doi: 10.1042/bj1640399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson L. S., Korner A. Influence of amino acid supply on ribosomes and protein synthesis of perfused rat liver. Biochem J. 1969 Mar;111(5):703–712. doi: 10.1042/bj1110703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khairallah E. A., Mortimore G. E. Assessment of protein turnover in perfused rat liver. Evidence for amino acid compartmentation from differential labeling of free and tRNA-gound valine. J Biol Chem. 1976 Mar 10;251(5):1375–1384. [PubMed] [Google Scholar]
- Kirschke H., Langner J., Wiederanders B., Ansorge S., Bohley P., Broghammer U. Intrazellulärer Proteinabbau. VII. Kathepsin L und H: Zwei neue Proteinasen aus Rattenleberlysosomen. Acta Biol Med Ger. 1976;35(3-4):285–299. [PubMed] [Google Scholar]
- Knowles S. E., Ballard F. J. Selective control of the degradation of normal and aberrant proteins in Reuber H35 hepatoma cells. Biochem J. 1976 Jun 15;156(3):609–617. doi: 10.1042/bj1560609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. Y., Krsmanovic V., Brawerman G. Attachment of ribosomes to membranes during polysome formation in mouse sarcoma 180 cells. J Cell Biol. 1971 Jun;49(3):683–691. doi: 10.1083/jcb.49.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGown E., Richardson A. G., Henderson L. M., Swan P. B. Effect of amino acids on ribosome aggregation and protein synthesis in perfused rat liver. J Nutr. 1973 Jan;103(1):109–116. doi: 10.1093/jn/103.1.109. [DOI] [PubMed] [Google Scholar]
- Meijer A. J., Gimpel J. A., Deleeuw G. A., Tager J. M., Williamson J. R. Role of anion translocation across the mitochondrial membrane in the regulation of urea synthesis from ammonia by isolated rat hepatocytes. J Biol Chem. 1975 Oct 10;250(19):7728–7738. [PubMed] [Google Scholar]
- Mortimore G. E., Woodside K. H., Henry J. E. Compartmentation of free valine and its relation to protein turnover in perfused rat liver. J Biol Chem. 1972 May 10;247(9):2776–2784. [PubMed] [Google Scholar]
- Munro H. N. Role of amino acid supply in regulating ribosome function. Fed Proc. 1968 Sep-Oct;27(5):1231–1237. [PubMed] [Google Scholar]
- Poole B. The kinetics of disappearance of labeled leucine from the free leucine pool of rat liver and its effect on the apparent turnover of catalase and other hepatic proteins. J Biol Chem. 1971 Nov;246(21):6587–6591. [PubMed] [Google Scholar]
- Pronczuk A. W., Rogers Q. R., Munro H. N. Liver polysome patterns of rats fed amino acid imbalanced diets. J Nutr. 1970 Nov;100(11):1249–1258. doi: 10.1093/jn/100.11.1249. [DOI] [PubMed] [Google Scholar]
- Righetti P., Little E. P., Wolf G. Reutilization of amino acids in protein synthesis in HeLa cells. J Biol Chem. 1971 Sep 25;246(18):5724–5732. [PubMed] [Google Scholar]
- Schreiber G., Urban J., Zähringer J., Reutter W., Frosch U. The secretion of serum protein and the synthesis of albumin and total protein in regenerating rat liver. J Biol Chem. 1971 Jul 25;246(14):4531–4538. [PubMed] [Google Scholar]
- Seglen P. O. Incorporation of radioactive amino acids into protein in isolated rat hepatocytes. Biochim Biophys Acta. 1976 Sep 6;442(3):391–404. doi: 10.1016/0005-2787(76)90313-0. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Inhibitor of protein degradation formed during incubation of isolated rat hepatocytes in a cell culture medium. Its identification as ammonia. Exp Cell Res. 1977 Jun;107(1):207–217. doi: 10.1016/0014-4827(77)90402-5. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of rat liver cells. 3. Enzymatic requirements for tissue dispersion. Exp Cell Res. 1973 Dec;82(2):391–398. doi: 10.1016/0014-4827(73)90357-1. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Protein degradation in isolated rat hepatocytes is inhibited by ammonia. Biochem Biophys Res Commun. 1975 Sep 2;66(1):44–52. doi: 10.1016/s0006-291x(75)80292-0. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Protein-catabolic stage of isolated rat hepatocytes. Biochim Biophys Acta. 1977 Jan 24;496(1):182–191. doi: 10.1016/0304-4165(77)90126-x. [DOI] [PubMed] [Google Scholar]
- Seglen P. O., Reith A. Ammonia inhibition of protein degradation in isolated rat hepatocytes. Quantitative ultrastructural alterations in the lysosomal system. Exp Cell Res. 1976 Jul;100(2):276–280. doi: 10.1016/0014-4827(76)90148-8. [DOI] [PubMed] [Google Scholar]
- Seglen P. O., Reith A. Ammonia inhibits protein secretion in isolated rat hepatocytes. Biochim Biophys Acta. 1977 Jan 24;496(1):29–35. doi: 10.1016/0304-4165(77)90112-x. [DOI] [PubMed] [Google Scholar]
- Seglen P. O., Solheim A. E. Valine uptake and incorporation into protein in isolated rat hepatocytes. Nature of the precursor pool for protein synthesis. Eur J Biochem. 1978 Apr;85(1):15–25. doi: 10.1111/j.1432-1033.1978.tb12208.x. [DOI] [PubMed] [Google Scholar]
- Sidransky H., Sarma D. S., Bongiorno M., Verney E. Effect of dietary tryptophan on hepatic polyribosomes and protein synthesis in fasted mice. J Biol Chem. 1968 Mar 25;243(6):1123–1132. [PubMed] [Google Scholar]
- Smulson M. E., Rideau C. Association of aminoacyl transferase II with ribosomes of intact HeLa cells during amino acid deprivation. J Biol Chem. 1970 Oct 25;245(20):5350–5353. [PubMed] [Google Scholar]
- Stubbs M., Krebs H. A. The accumulation of aspartate in the presence of ethanol in rat liver. Biochem J. 1975 Jul;150(1):41–45. doi: 10.1042/bj1500041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ventrooij W. J., Henshaw E. C., Hirsch C. A. Nutritional effects on the polyribosome distribution and rate of protein synthesis in Ehrlich ascites tumor cells in culture. J Biol Chem. 1970 Nov 25;245(22):5947–5953. [PubMed] [Google Scholar]
- VanDenBorre M., Webb T. E. Perfusate composition and stability of polyribosomes in perfused liver. Life Sci II. 1972 Apr 8;11(7):347–354. doi: 10.1016/0024-3205(72)90074-4. [DOI] [PubMed] [Google Scholar]
- Weigand K., Warnze H., Falge C. Synthesis of angiotensinogen by isolated rat liver cells and its regulation in comparison to serum albumin. Biochem Biophys Res Commun. 1977 Mar 7;75(1):102–110. doi: 10.1016/0006-291x(77)91295-5. [DOI] [PubMed] [Google Scholar]
- Woodside K. H. Effects of cycloheximide on protein degradation and gluconeogenesis in the perfused rat liver. Biochim Biophys Acta. 1976 Jan 14;421(1):70–79. doi: 10.1016/0304-4165(76)90170-7. [DOI] [PubMed] [Google Scholar]
- Woodside K. H., Ward W. F., Mortimore G. E. Effects of glucagon on general protein degradation and synthesis in perfused rat liver. J Biol Chem. 1974 Sep 10;249(17):5458–5463. [PubMed] [Google Scholar]
- Zahlten R. N., Kneer N. M., Stratman F. W., Lardy H. A. The influence of ammonium and calcium lons on gluconeogenesis in isolated rat hepatocytes and their response to glucagon and epinephrine. Arch Biochem Biophys. 1974 Apr 2;161(2):528–535. doi: 10.1016/0003-9861(74)90335-x. [DOI] [PubMed] [Google Scholar]
- Zaleski J., Bryla J. Effects of oleate, palmitate, and octanoate on gluconeogenesis in isolated rabbit liver cells. Arch Biochem Biophys. 1977 Oct;183(2):553–562. doi: 10.1016/0003-9861(77)90390-3. [DOI] [PubMed] [Google Scholar]
- van Venrooij W. J., Henshaw E. C., Hirsch C. A. Effects of deprival of glucose or individual amino acids on polyribosome distribution and rate of protein synthesis in cultured mammalian cells. Biochim Biophys Acta. 1972 Jan 18;259(1):127–137. doi: 10.1016/0005-2787(72)90480-7. [DOI] [PubMed] [Google Scholar]


