Abstract
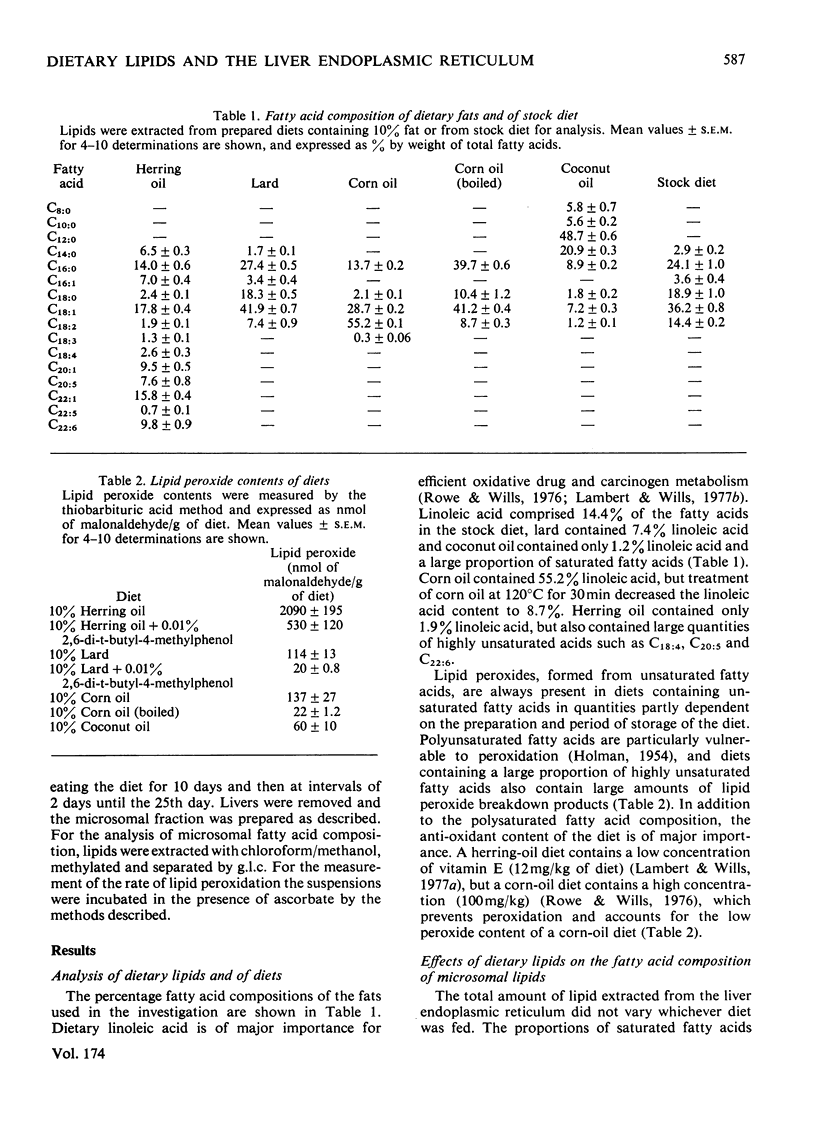
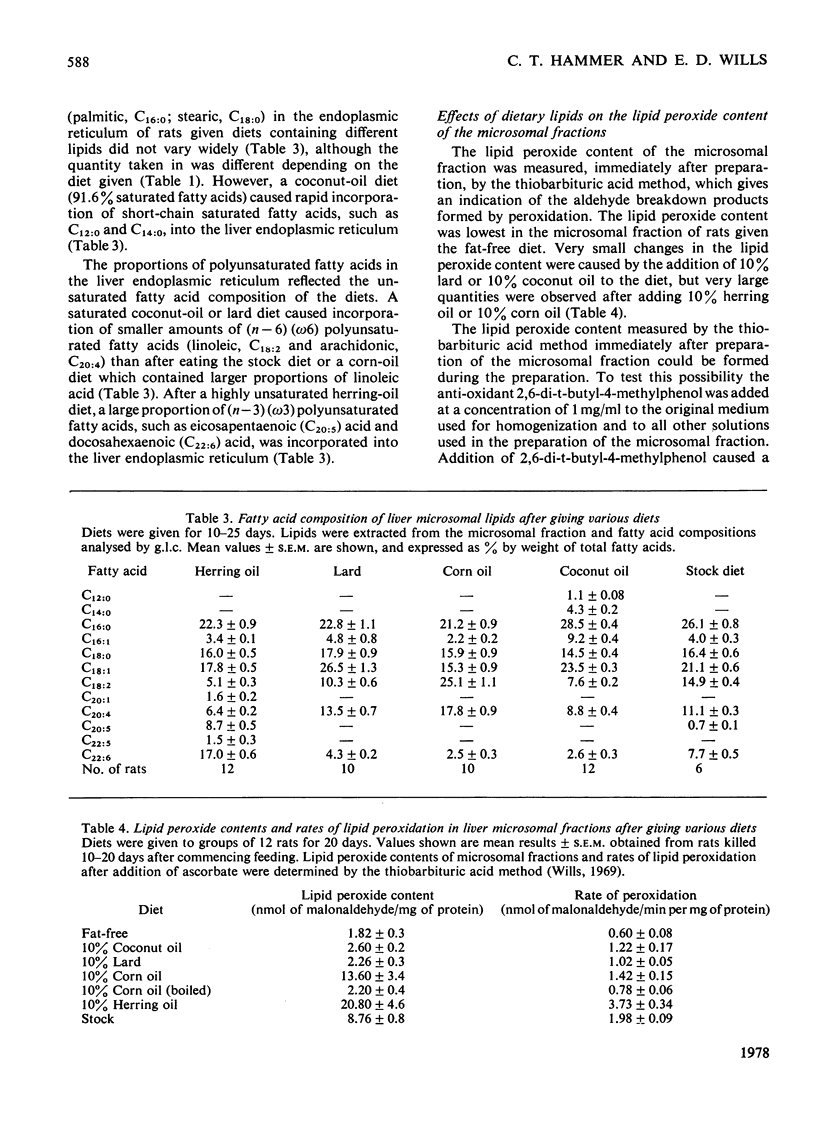
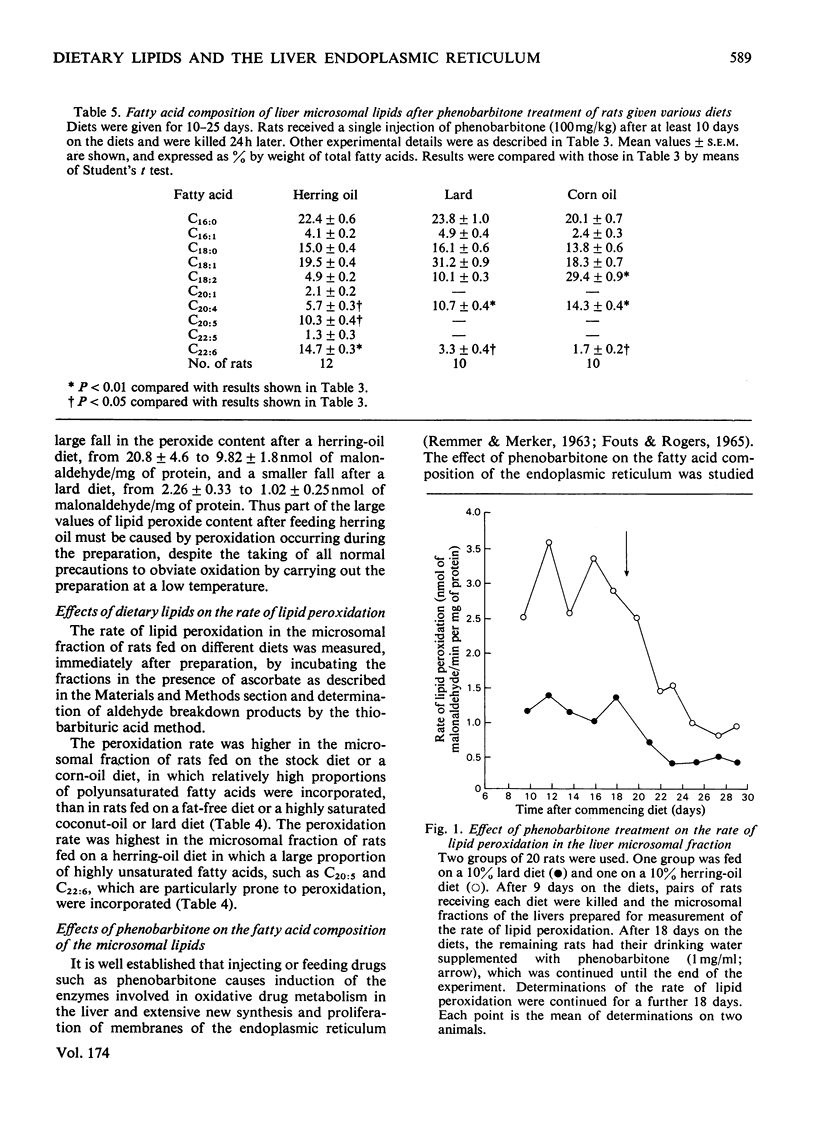
The fatty acid compositions of the lipids and the lipid peroxide concentrations and rates of lipid peroxidation were determined in suspensions of liver endoplasmic reticulum isolated from rats fed on synthetic diets in which the fatty acid composition had been varied but the remaining constituents (protein, carbohydrate, vitamins and minerals) kept constant. Stock diet and synthetic diets containing no fat, 10% corn oil, herring oil, coconut oil or lard were used. The fatty acid composition of the liver endoplasmic reticulum lipid was markedly dependent on the fatty acid composition of the dietary lipid. Feeding a herring-oil diet caused incorporation of 8.7% eicosapentaenoic acid (C20:5) and 17% docosahexaenoic acid (C22:6), but only 5.1% linoleic acid (C18:2) and 6.4% arachidonic acid (C20:4), feeding a corn-oil diet caused incorporation of 25.1% C18:2, 17.8% C20:4 and 2.5% C22:6 fatty acids, and feeding a lard diet caused incorporation of 10.3% C18:2, 13.5% C20:4 and 4.3% C22:6 fatty acids into the liver endoplasmic-reticulum lipids. Phenobarbitone injection (100mg/kg) decreased the incorporation of C20:4 and C22:6 fatty acids into the liver endoplasmic reticulum of rats fed on a lard, corn-oil or herring-oil diet. Microsomal lipid peroxide concentrations and rates of peroxidation in the presence of ascorbate depended on the nature and quantity of the polyunsaturated fatty acids in the diet. The lipid peroxide content was 1.82±0.30nmol of malonaldehyde/mg of protein and the rate of peroxidation was 0.60±0.08nmol of malonaldehyde/min per mg of protein after feeding a fat-free diet, and the values were increased to 20.80nmol of malonaldehyde/mg of protein and 3.73nmol of malonaldehyde/min per mg of protein after feeding a 10% herring-oil diet in which polyunsaturated fatty acids formed 24% of the total fatty acids. Addition of α-tocopherol to the diets (120mg/kg of diet) caused a very large decrease in the lipid peroxide concentration and rate of lipid peroxidation in the endoplasmic reticulum, but addition of the synthetic anti-oxidant 2,6-di-t-butyl-4-methylphenol to the diet (100mg/kg of diet) was ineffective. Treatment of the animals with phenobarbitone (1mg/ml of drinking water) caused a sharp fall in the rate of lipid peroxidation. It is concluded that the polyunsaturated fatty acid composition of the diet regulates the fatty acid composition of the liver endoplasmic reticulum, and this in turn is an important factor controlling the rate and extent of lipid peroxidation in vitro and possibly in vivo.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDREWS J. S., GRIFFITH W. H., MEAD J. F., STEIN R. A. Toxicity of air-oxidized soybean oil. J Nutr. 1960 Feb;70:199–210. doi: 10.1093/jn/70.2.199. [DOI] [PubMed] [Google Scholar]
- Alfin-Slater R. B., Aftergood L. Essential fatty acids reinvestigated. Physiol Rev. 1968 Oct;48(4):758–784. doi: 10.1152/physrev.1968.48.4.758. [DOI] [PubMed] [Google Scholar]
- Cook L. J., Scott T. W., Ferguson K. A., McDonald I. W. Production of poly-unsaturated ruminant body fats. Nature. 1970 Oct 10;228(5267):178–179. doi: 10.1038/228178a0. [DOI] [PubMed] [Google Scholar]
- DIPLOCK A. T., BUNYAN J., GREEN J., EDWIN E. E. Studies on vitamin E. 7. The effect of thiamine, riboflavin and pantothenic acid on ubiquinone and ubichromenol in the rat. Biochem J. 1961 Apr;79:105–108. doi: 10.1042/bj0790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison S. C., Wills E. D. Studies on the lipid composition of the rat liver endoplasmic reticulum after induction with phenobarbitone and 20-methylcholanthrene. Biochem J. 1974 Jun;140(3):461–468. doi: 10.1042/bj1400461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOUTS J. R., ROGERS L. A. MORPHOLOGICAL CHANGES IN THE LIVER ACCOMPANYING STIMULATION OF MICROSOMAL DRUG METABOLIZING ENZYME ACTIVITY BY PHENOBARBITAL, CHLORDANE, BENZPYRENE OR METHYL-CHOLANTHRENE IN RATS. J Pharmacol Exp Ther. 1965 Jan;147:112–119. [PubMed] [Google Scholar]
- Kamath S. A., Rubin E. Interaction of calcium with microsomes: a modified method for the rapid isolation of rat liver microsomes. Biochem Biophys Res Commun. 1972 Oct 6;49(1):52–59. doi: 10.1016/0006-291x(72)90008-3. [DOI] [PubMed] [Google Scholar]
- Lambert L., Wills E. D. The effect of dietary lipid peroxides, sterols and oxidised sterols on cytochrome P450 and oxidative demethylation in the endoplasmic reticulum. Biochem Pharmacol. 1977 Aug 1;26(15):1417–1421. doi: 10.1016/0006-2952(77)90367-7. [DOI] [PubMed] [Google Scholar]
- Lambert L., Wills E. D. The effect of dietary lipids on 3, 4, benzo[a]pyrene metabolism in the hepatic endoplasmic reticulum. Biochem Pharmacol. 1977 Aug 1;26(15):1423–1427. doi: 10.1016/0006-2952(77)90368-9. [DOI] [PubMed] [Google Scholar]
- MORRISON W. R., SMITH L. M. PREPARATION OF FATTY ACID METHYL ESTERS AND DIMETHYLACETALS FROM LIPIDS WITH BORON FLUORIDE--METHANOL. J Lipid Res. 1964 Oct;5:600–608. [PubMed] [Google Scholar]
- Marshall W. J., McLean A. E. A requirement for dietary lipids for induction of cytochrome P-450 by phenobarbitone in rat liver microsomal fraction. Biochem J. 1971 May;122(4):569–573. doi: 10.1042/bj1220569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May H. E., McCay P. B. Reduced triphosphopyridine nucleotide oxidase-catalyzed alterations of membrane phospholipids. I. Nature of the lipid alterations. J Biol Chem. 1968 May 10;243(9):2288–2295. [PubMed] [Google Scholar]
- May H. E., McCay P. B. Reduced triphosphopyridine nucleotide oxidase-catalyzed alterations of membrane phospholipids. II. Enzymic properties and stoichiometry. J Biol Chem. 1968 May 10;243(9):2296–2305. [PubMed] [Google Scholar]
- Norred W. P., Wade A. E. Dietary fatty acid-induced alterations of hepatic microsomal drug metabolism. Biochem Pharmacol. 1972 Nov 1;21(21):2887–2897. doi: 10.1016/0006-2952(72)90213-4. [DOI] [PubMed] [Google Scholar]
- Norred W. P., Wade A. E. Effect of dietary lipid ingestion on the induction of drug-metabolizing enzymes by phenobarbital. Biochem Pharmacol. 1973 Feb 1;22(3):432–436. doi: 10.1016/0006-2952(73)90425-5. [DOI] [PubMed] [Google Scholar]
- REMMER H., MERKER H. J. DRUG-INDUCED CHANGES IN THE LIVER ENDOPLASMIC RETICULUM: ASSOCIATION WITH DRUG-METABOLIZING ENZYMES. Science. 1963 Dec 27;142(3600):1657–1658. doi: 10.1126/science.142.3600.1657. [DOI] [PubMed] [Google Scholar]
- Rowe L., Wills E. D. The effect of dietary lipids and vitamin E on lipid peroxide formation, cytochrome P-450 and oxidative demethylation in the endoplasmic reticulum. Biochem Pharmacol. 1976 Jan 15;25(2):175–179. doi: 10.1016/0006-2952(76)90287-2. [DOI] [PubMed] [Google Scholar]
- Victoria E. J., Barber A. A. Peroxidation of microsomal membrane protein--lipid complexes. Lipids. 1969 Nov;4(6):582–588. doi: 10.1007/BF02531045. [DOI] [PubMed] [Google Scholar]
- WILLS E. D. THE EFFECT OF INORGANIC IRON ON THE THIOBARBITURIC ACID METHOD FOR THE DETERMINATION OF LIPID PEROXIDES. Biochim Biophys Acta. 1964 Aug 5;84:475–477. doi: 10.1016/0926-6542(64)90016-2. [DOI] [PubMed] [Google Scholar]
- Wasserman R. H., Taylor A. N. Metabolic roles of fat-soluble vitamins D, E, and K. Annu Rev Biochem. 1972;41:179–202. doi: 10.1146/annurev.bi.41.070172.001143. [DOI] [PubMed] [Google Scholar]
- Wills E. D. Lipid peroxide formation in microsomes. General considerations. Biochem J. 1969 Jun;113(2):315–324. doi: 10.1042/bj1130315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills E. D. Mechanisms of lipid peroxide formation in animal tissues. Biochem J. 1966 Jun;99(3):667–676. doi: 10.1042/bj0990667. [DOI] [PMC free article] [PubMed] [Google Scholar]


