Abstract
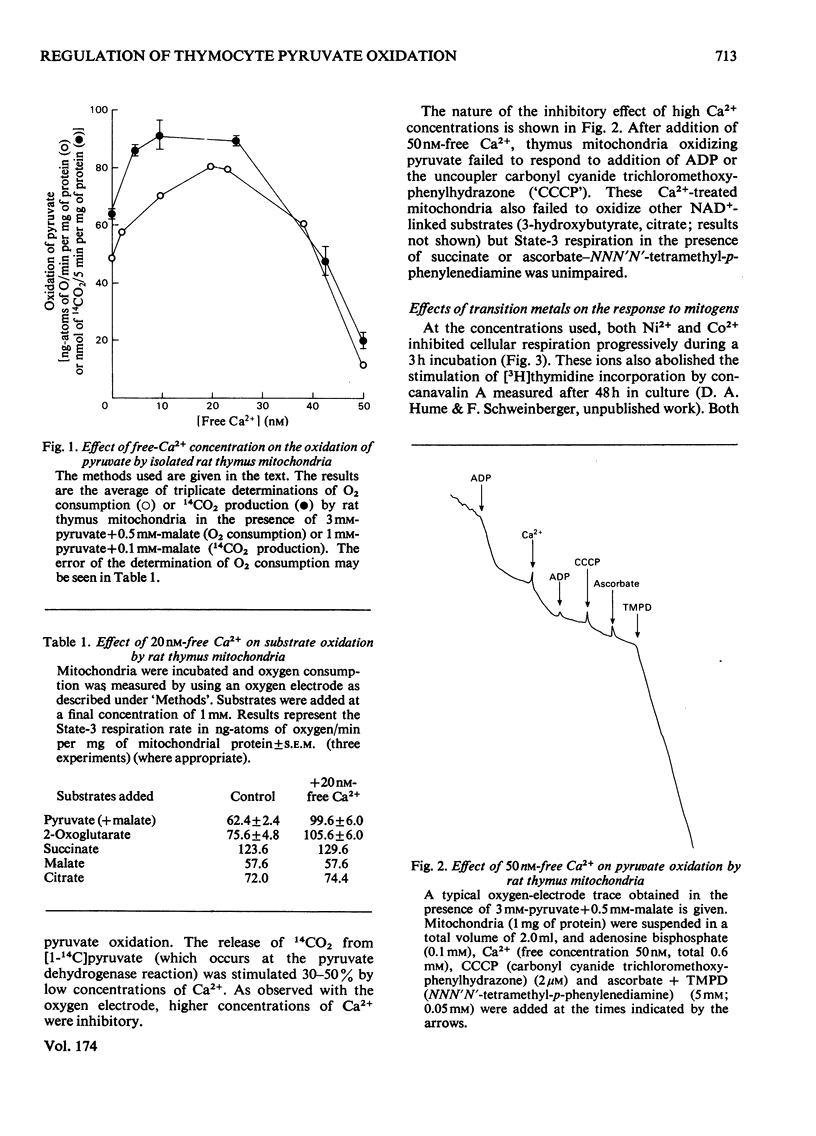
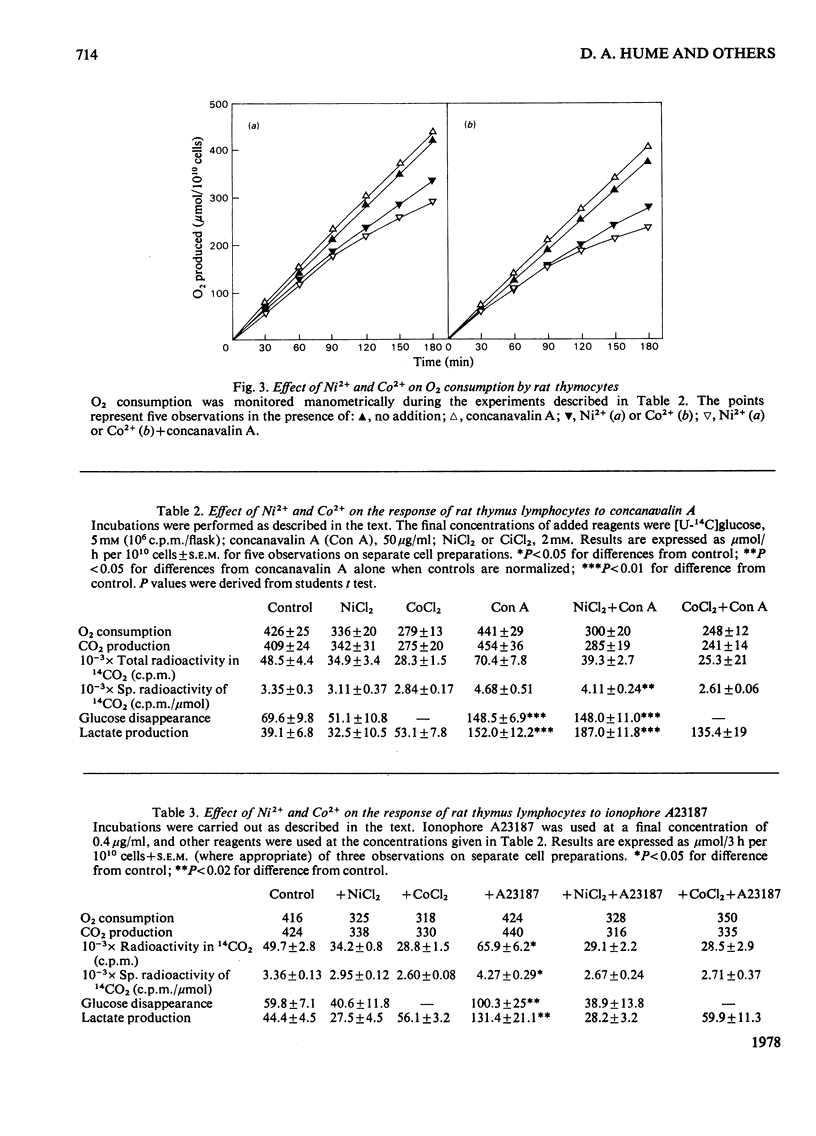
1. Calcium concentrations in the nanomolar range cause a specific stimulation of the oxidation of pyruvate by isolated mitochondria from rat thymus that is sufficient to account precisely for the stimulation of pyruvate oxidation observed when rat thymocytes are incubated with the mitogens concanavalin A or ionophore A23187. 2. Higher concentrations of Ca2+ (more than 50 nM) inhibit the oxidation of NAD+-linked substrates by rat thymus mitochondria without affecting the oxidation of succinate or ascorbate+ NNN'N'-tetramethyl-p-phenylendiamine. 3. The addition of Ni2+ or Co2+ (2mM) to rat thymocytes prevents the response to concanavalin A at the level of pyruvate oxidation without affecting the stimulation of glycolysis induced by this mitogen. In contrast, the complete metabolic response to the ionophore A23187 is abolished by these ions. Ni2+ and Co2+ interfere with the ability of the ionophore to transport Ca2+ across the plasma membrane. 4. Concanavalin A, but not ionophore A23187, increases the respiratory inhibition induced by Ni2+ and Co2+. 5. These results support the view that mitogens stimulate lymphocyte pyruvate oxidation through an increase in cellular Ca2+ uptake.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Averdunk R. Early changes of 'leak flux' and the cation content of lymphocytes by concanavalin A. Biochem Biophys Res Commun. 1976 May 3;70(1):101–109. doi: 10.1016/0006-291x(76)91114-1. [DOI] [PubMed] [Google Scholar]
- CHANCE B. THE ENERGY-LINKED REACTION OF CALCIUM WITH MITOCHONDRIA. J Biol Chem. 1965 Jun;240:2729–2748. [PubMed] [Google Scholar]
- Culvenor J. G., Weidemann M. J. Phytohaemagglutinin stimulation of rat thymus lymphocytes glycolysis. Biochim Biophys Acta. 1976 Jul 21;437(2):354–363. doi: 10.1016/0304-4165(76)90005-2. [DOI] [PubMed] [Google Scholar]
- Denton R. M., Randle P. J., Bridges B. J., Cooper R. H., Kerbey A. L., Pask H. T., Severson D. L., Stansbie D., Whitehouse S. Regulation of mammalian pyruvate dehydrogenase. Mol Cell Biochem. 1975 Oct 31;9(1):27–53. doi: 10.1007/BF01731731. [DOI] [PubMed] [Google Scholar]
- Fisher A. B., Scarpa A., LaNoue K. F., Bassett D., Williamson J. R. Respiration of rat lung mitochondria and the influence of Ca 2+ on substrate utilization. Biochemistry. 1973 Mar 27;12(7):1438–1445. doi: 10.1021/bi00731a026. [DOI] [PubMed] [Google Scholar]
- Freedman M. H., Raff M. C. Induction of increased calcium uptake in mouse T lymphocytes by concanavalin A and its modulation by cyclic nucleotides. Nature. 1975 May 29;255(5507):378–382. doi: 10.1038/255378a0. [DOI] [PubMed] [Google Scholar]
- Hesketh T. R., Smith G. A., Houslay M. D., Warren G. B., Metcalfe J. C. Is an early calcium flux necessary to stimulate lymphocytes? Nature. 1977 Jun 9;267(5611):490–494. doi: 10.1038/267490a0. [DOI] [PubMed] [Google Scholar]
- Hume D. A., Radik J. L., Ferber E., Weidemann M. J. Aerobic glycolysis and lymphocyte transformation. Biochem J. 1978 Sep 15;174(3):703–709. doi: 10.1042/bj1740703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A., De Gasquet P. Inhibition of gluconeogenesis by alpha-oxo acids. Biochem J. 1964 Jan;90(1):149–154. doi: 10.1042/bj0900149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn T. C., Pettit F. H., Hucho F., Reed L. J. Alpha-keto acid dehydrogenase complexes. XI. Comparative studies of regulatory properties of the pyruvate dehydrogenase complexes from kidney, heart, and liver mitochondria. Proc Natl Acad Sci U S A. 1969 Sep;64(1):227–234. doi: 10.1073/pnas.64.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn T. C., Pettit F. H., Reed L. J. Alpha-keto acid dehydrogenase complexes. X. Regulation of the activity of the pyruvate dehydrogenase complex from beef kidney mitochondria by phosphorylation and dephosphorylation. Proc Natl Acad Sci U S A. 1969 Jan;62(1):234–241. doi: 10.1073/pnas.62.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckasen J. R., White J. G., Kersey J. H. Mitogenic properties of a calcium ionophore, A23187. Proc Natl Acad Sci U S A. 1974 Dec;71(12):5088–5090. doi: 10.1073/pnas.71.12.5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maino V. C., Green N. M., Crumpton M. J. The role of calcium ions in initiating transformation of lymphocytes. Nature. 1974 Sep 27;251(5473):324–327. doi: 10.1038/251324b0. [DOI] [PubMed] [Google Scholar]
- Parker C. W. Correlation between mitogenicity and stimulation of calcium uptake in human lymphocytes. Biochem Biophys Res Commun. 1974 Dec 23;61(4):1180–1186. doi: 10.1016/s0006-291x(74)80408-0. [DOI] [PubMed] [Google Scholar]
- Reed K. C. An oxygen polarograph designed for undergraduate use. Anal Biochem. 1972 Nov;50(1):206–212. doi: 10.1016/0003-2697(72)90500-3. [DOI] [PubMed] [Google Scholar]
- Reed P. W., Lardy H. A. A23187: a divalent cation ionophore. J Biol Chem. 1972 Nov 10;247(21):6970–6977. [PubMed] [Google Scholar]
- Reeves J. P. Calcium-dependent stimulation of 3-O-methylglucose uptake in rat thymocytes by the divalent cation ionophore A23187. J Biol Chem. 1975 Dec 25;250(24):9428–9430. [PubMed] [Google Scholar]
- Schuster S. M., Olson M. S. The regulation of pyruvate dehydrogenase in isolated beef heart mitochondria. The role of calcium, magnesium, and permeant anions. J Biol Chem. 1974 Nov 25;249(22):7159–7165. [PubMed] [Google Scholar]
- Severson D. L., Denton R. M., Pask H. T., Randle P. J. Calcium and magnesium ions as effectors of adipose-tissue pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1974 May;140(2):225–237. doi: 10.1042/bj1400225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter D., Weidemann M. J. Regulation of carbohydrate metabolism in lymphoid tissue. Quantitative aspects of [U-14C]glucose oxidation by rat spleen slices. Biochem J. 1975 Jun;148(3):583–594. doi: 10.1042/bj1480583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar E. K., Weidemann M. J. Location of an oligomycin-insensitive and magnesium ion-stimulated adenosine triphosphatase in rat spleen mitochondria. Biochem J. 1976 Nov 15;160(2):383–393. doi: 10.1042/bj1600383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. L., McClain D. A., Edelman G. M. Modulation of lymphocyte mitogenesis. Proc Natl Acad Sci U S A. 1975 May;72(5):1917–1921. doi: 10.1073/pnas.72.5.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitesell R. R., Johnson R. A., Tarpley H. L., Regen D. M. Mitogen-stimulated glucose transport in thymocytes. Possible role of Ca++ and antagonism by adenosine 3':5'-monophosphate. J Cell Biol. 1977 Feb;72(2):456–469. doi: 10.1083/jcb.72.2.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasmeen D., Laird A. J., Hume D. A., Weidemann M. J. Activation of 3-O-methyl-glucose transport in rat thymus lymphocytes by concanavalin A. Temperature and calcium ion dependence and sensitivity to puromycin but to cycloheximide. Biochim Biophys Acta. 1977 Nov 7;500(1):89–102. doi: 10.1016/0304-4165(77)90049-6. [DOI] [PubMed] [Google Scholar]


