Abstract
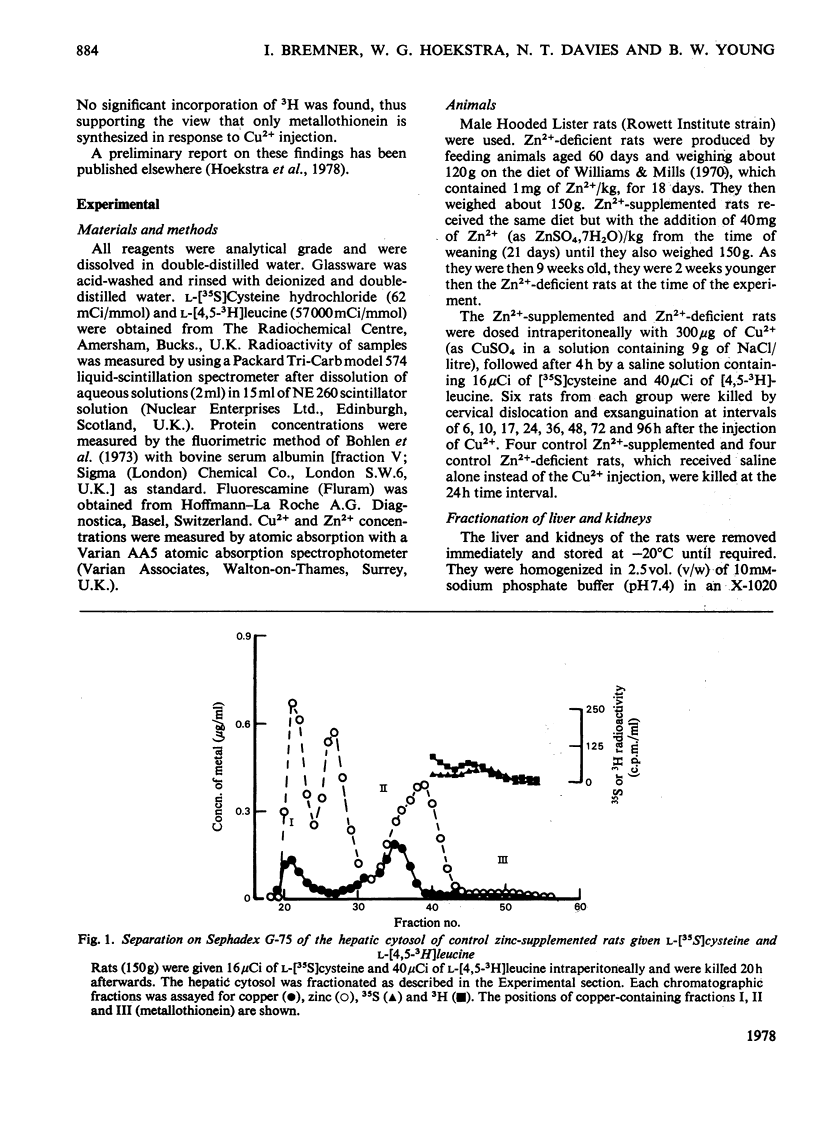
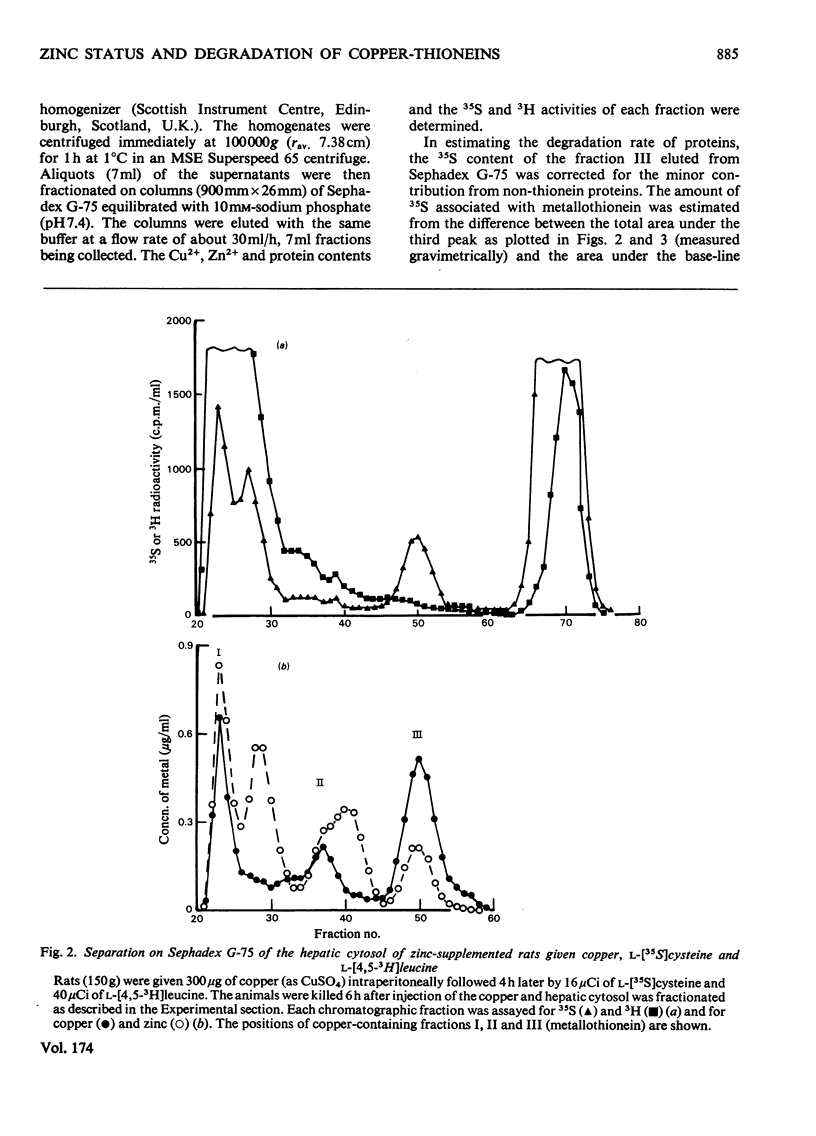
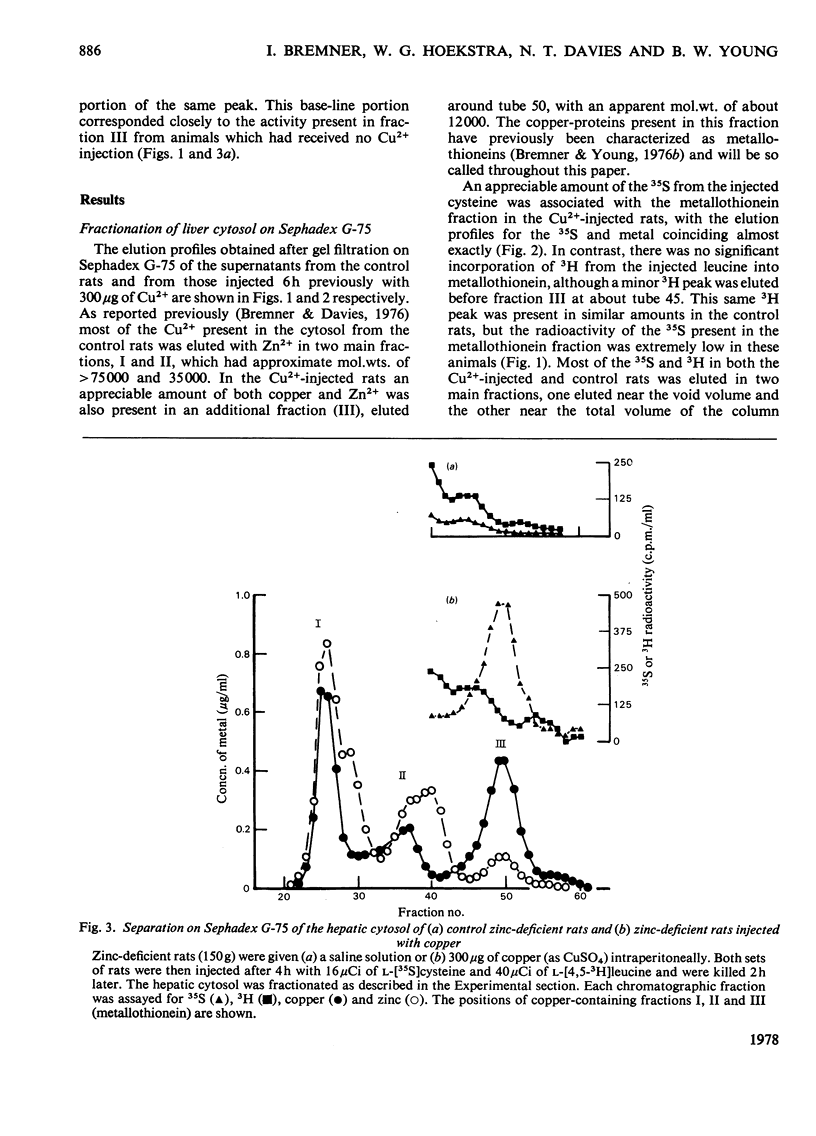
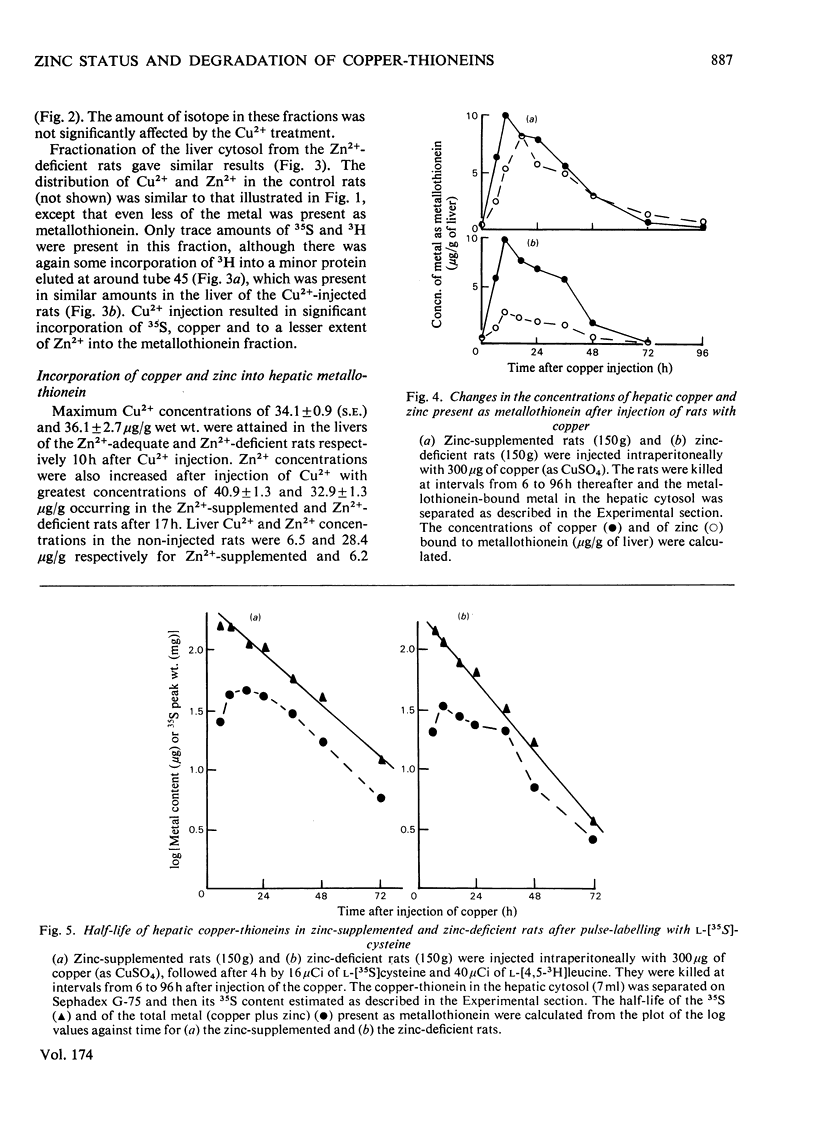
Injection of Zn2+-adequate and Zn2+-deficient rats with Cu2+ stimulated the incorporation of l-[35S]cysteine into a low-molecular-weight Cu2+-binding protein in both liver and kidney. No significant incorporation of l-[4,5-3H]leucine into this protein occurred, confirming the previous claim that it was metallothionein and not some other leucine-rich protein. The half-life of the protein was found to be 16.9 +/- 1.0 (S.E.)h in the liver of Zn2+-adequate rats but only 12.3 +/- 0.5h in Zn2+-deficient animals. The degradation rate of the metallothionein was similar to the rate of disappearance of Cu2+ and Zn2+ from the protein, indicating that the release of mental from the protein and its catabolism occurred simultaneously. There was no significant difference in the half-lives of the hepatic or renal copper-thioneins in Zn2+-adequate rats.
Full text
PDF
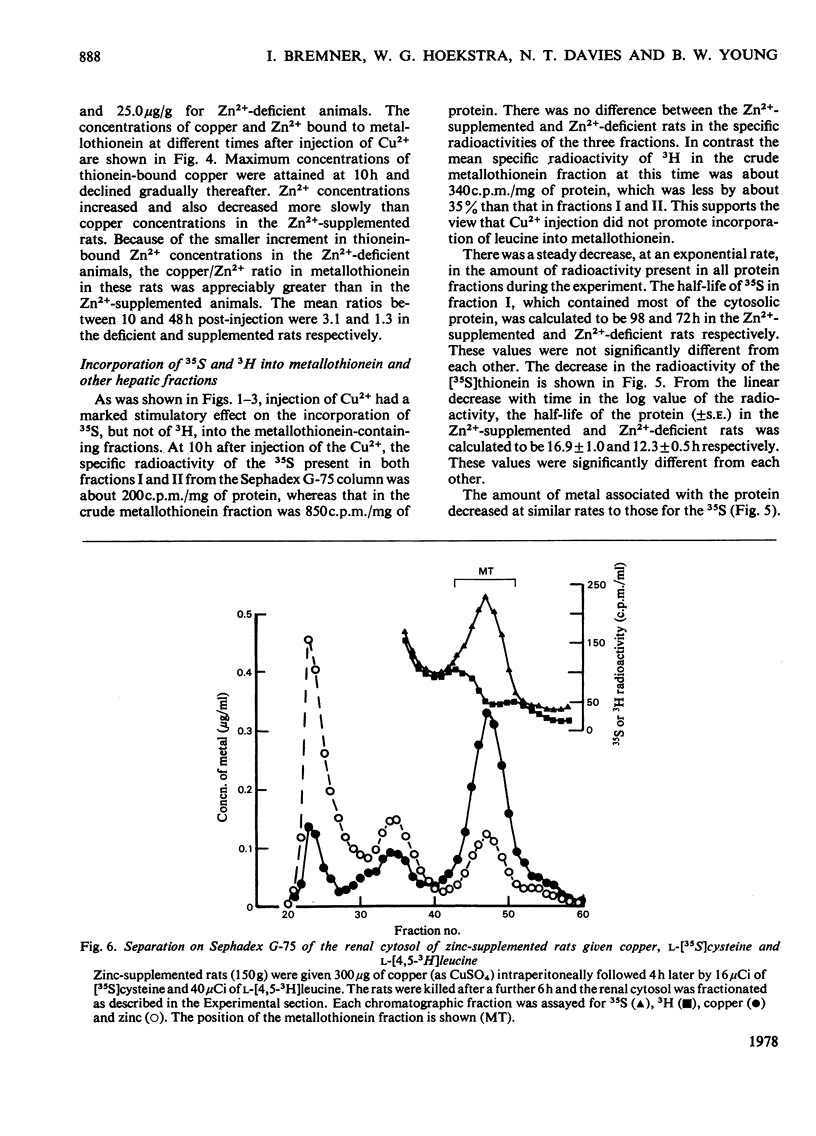
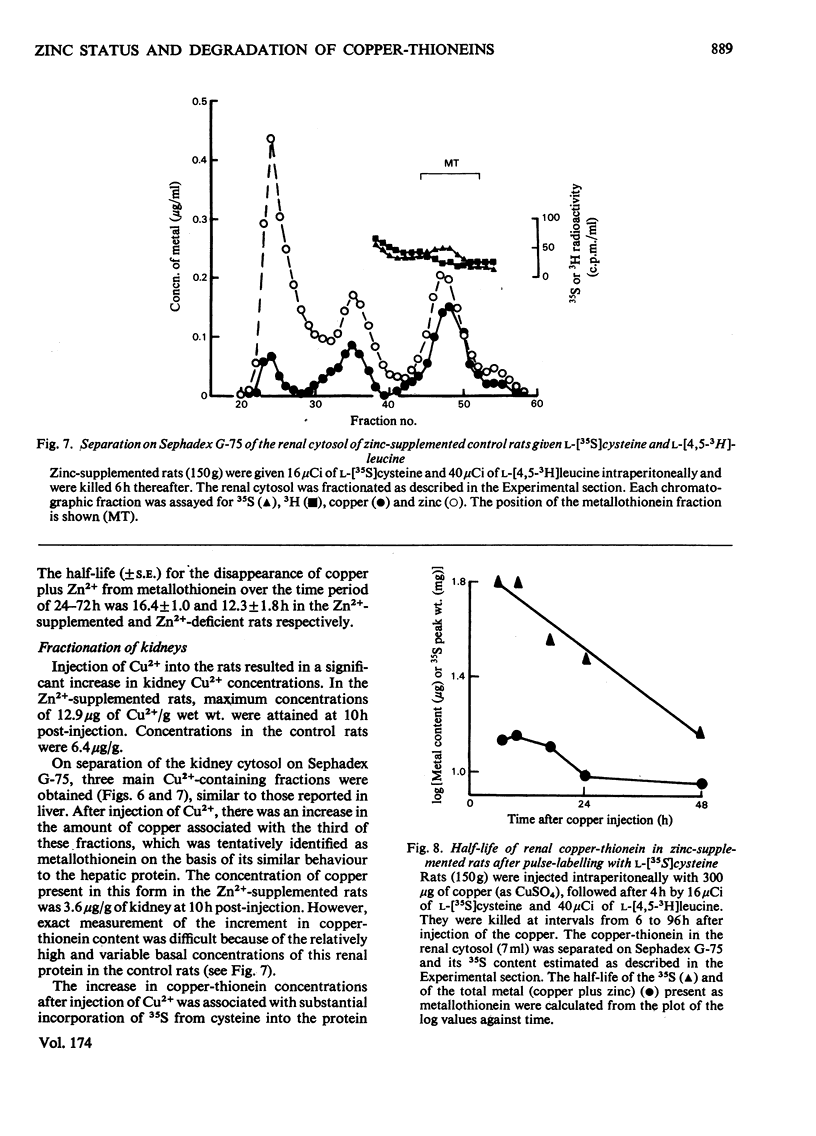
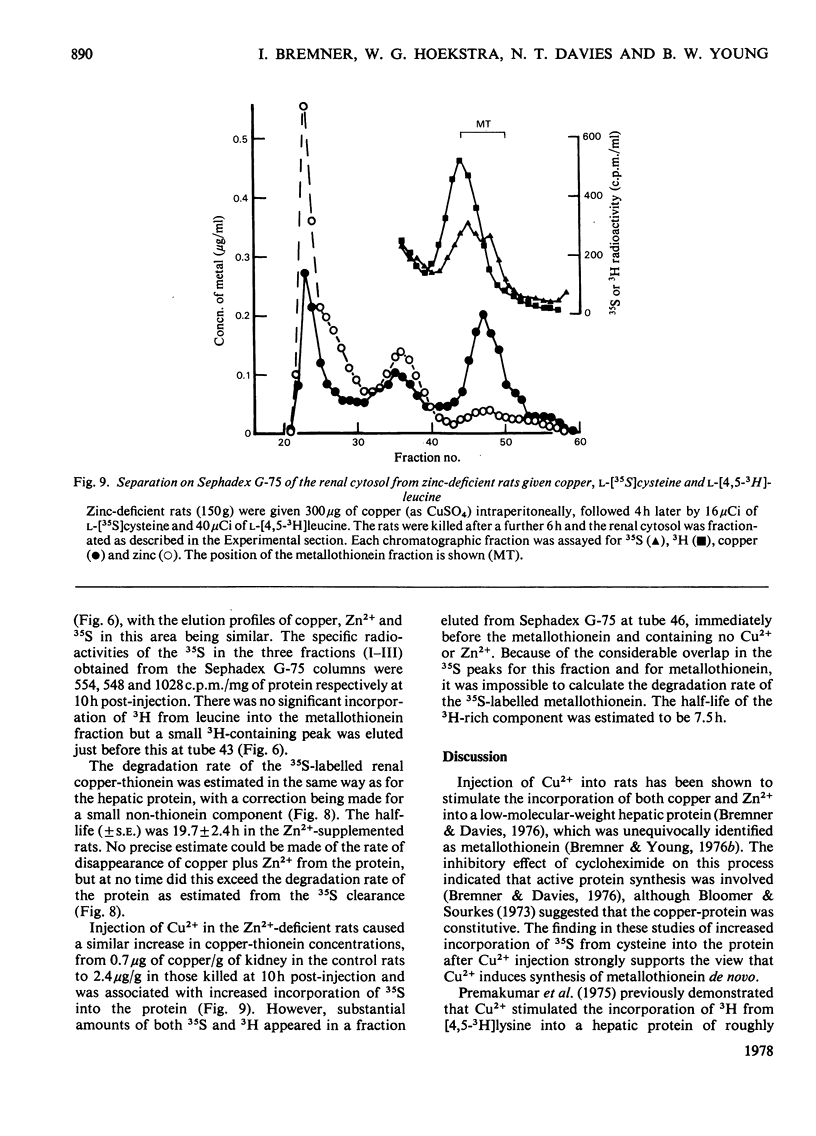


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloomer L. C., Sourkes T. L. The effect of copper loading on the distribution of copper in rat liver cytosol. Biochem Med. 1973 Aug;8(1):78–91. doi: 10.1016/0006-2944(73)90011-2. [DOI] [PubMed] [Google Scholar]
- Bremner I., Davies N. T. Studies on the appearance of a hepatic copper-binding protein in normal and zinc-deficient rats. Br J Nutr. 1976 Jul;36(1):101–112. doi: 10.1079/bjn19760061. [DOI] [PubMed] [Google Scholar]
- Bremner I., Davies N. T. The induction of metallothionein in rat liver by zinc injection and restriction of food intake. Biochem J. 1975 Sep;149(3):733–738. doi: 10.1042/bj1490733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner I. The relationship between the zinc status of pigs and the occurrence of copper- and Zn-binding proteins in liver. Br J Nutr. 1976 Mar;35(2):245–252. doi: 10.1079/bjn19760028. [DOI] [PubMed] [Google Scholar]
- Bremner I., Young B. W. Copper thionein in the kidneys of copper-poisoned sheep. Chem Biol Interact. 1977 Oct;19(1):13–23. doi: 10.1016/0009-2797(77)90039-4. [DOI] [PubMed] [Google Scholar]
- Bremner I., Young B. W. Isolation of (copper, zinc)-thioneins from the livers of copper-injected rats. Biochem J. 1976 Aug 1;157(2):517–520. doi: 10.1042/bj1570517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner I., Young B. W. Isolation of (copper, zinc-) thioneins from pig liver. Biochem J. 1976 Jun 1;155(3):631–635. doi: 10.1042/bj1550631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhlen P., Stein S., Dairman W., Udenfriend S. Fluorometric assay of proteins in the nanogram range. Arch Biochem Biophys. 1973 Mar;155(1):213–220. doi: 10.1016/s0003-9861(73)80023-2. [DOI] [PubMed] [Google Scholar]
- Chen R. W., Whanger P. D., Weswig P. H. Biological function of metallothionein. I. Synthesis and degradation of rat liver metallothionein. Biochem Med. 1975 Feb;12(2):95–105. doi: 10.1016/0006-2944(75)90100-3. [DOI] [PubMed] [Google Scholar]
- Chvapil M. New aspects in the biological role of zinc: a stabilizer of macromolecules and biological membranes. Life Sci. 1973 Oct 16;13(8):1041–1049. doi: 10.1016/0024-3205(73)90372-x. [DOI] [PubMed] [Google Scholar]
- Drum D. E., Harrison J. H., 4th, Li T. K., Bethune J. L., Vallee B. L. Structural and functional zinc in horse liver alcohol dehydrogenase. Proc Natl Acad Sci U S A. 1967 May;57(5):1434–1440. doi: 10.1073/pnas.57.5.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drysdale J. W., Munro H. N. Regulation of synthesis and turnover of ferritin in rat liver. J Biol Chem. 1966 Aug 10;241(15):3630–3637. [PubMed] [Google Scholar]
- Evans G. W. Copper homeostasis in the mammalian system. Physiol Rev. 1973 Jul;53(3):535–570. doi: 10.1152/physrev.1973.53.3.535. [DOI] [PubMed] [Google Scholar]
- Feldman S. L., Cousins R. J. Degradation of hepatic zinc-thionein after parenteral zinc administration. Biochem J. 1976 Dec 15;160(3):583–588. doi: 10.1042/bj1600583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass R. D., Doyle D. On the measurement of protein turnover in animal cells. J Biol Chem. 1972 Aug 25;247(16):5234–5242. [PubMed] [Google Scholar]
- Goldberg A. L., Dice J. F. Intracellular protein degradation in mammalian and bacterial cells. Annu Rev Biochem. 1974;43(0):835–869. doi: 10.1146/annurev.bi.43.070174.004155. [DOI] [PubMed] [Google Scholar]
- Hartmann H. J., Weser U. Copper-thionein from fetal bovine liver. Biochim Biophys Acta. 1977 Mar 28;491(1):211–222. doi: 10.1016/0005-2795(77)90057-5. [DOI] [PubMed] [Google Scholar]
- Irons R. D., Smith J. C. Isolation of a non-thionein copper-binding protein from liver of copper-injected rats. Chem Biol Interact. 1977 Jul;18(1):83–89. doi: 10.1016/0009-2797(77)90143-0. [DOI] [PubMed] [Google Scholar]
- Madapallimatam G., Riordan J. R. Antibodies to low molecular weight copper binding protein from liver. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1286–1293. doi: 10.1016/s0006-291x(77)80119-8. [DOI] [PubMed] [Google Scholar]
- PISCATOR M. OM KADMIUM I NORMALA MAENNISKONJURAR SAMT REDOGOERELSE FOER ISOLERING AV METALLOTHIONEIN UR LEVER FRAN KADMIUMEXPONERADE KANINER. Nord Hyg Tidskr. 1964;45:76–82. [PubMed] [Google Scholar]
- Premakumar R., Winge D. R., Wiley R. D., Rajagopalan K. V. Copper-induced synthesis of copper-chelatin in rat liver. Arch Biochem Biophys. 1975 Sep;170(1):267–277. doi: 10.1016/0003-9861(75)90117-4. [DOI] [PubMed] [Google Scholar]
- Richards M. P., Cousins R. J. Influence of parenteral zinc and actinomycin D on tissue zinc uptake and the synthesis of a zinc - binding protein. Bioinorg Chem. 1975 Apr;4(3):215–224. doi: 10.1016/s0006-3061(00)80104-0. [DOI] [PubMed] [Google Scholar]
- Richards M. P., Cousins R. J. Metallothionein and its relationship to the metabolism of dietary zinc in rats. J Nutr. 1976 Nov;106(11):1591–1599. doi: 10.1093/jn/106.11.1591. [DOI] [PubMed] [Google Scholar]
- Riordan J. R., Gower I. Purification of low molecular weight copper proteins from copper loaded liver. Biochem Biophys Res Commun. 1975 Sep 16;66(2):678–686. doi: 10.1016/0006-291x(75)90563-x. [DOI] [PubMed] [Google Scholar]
- Shaikh Z. A., Smith J. C. The biosynthesis of metallothionein rat liver and kidney after administration of cadmium. Chem Biol Interact. 1976 Dec;15(4):327–336. doi: 10.1016/0009-2797(76)90138-1. [DOI] [PubMed] [Google Scholar]
- Williams R. B., Mills C. F. The experimental production of zinc deficiency in the rat. Br J Nutr. 1970 Dec;24(4):989–1003. doi: 10.1079/bjn19700102. [DOI] [PubMed] [Google Scholar]
- Winge D. R., Premakumar R., Wiley R. D., Rajagopalan K. V. Copper-chelatin: purification and properties of a copper-binding protein from rat liver. Arch Biochem Biophys. 1975 Sep;170(1):253–266. doi: 10.1016/0003-9861(75)90116-2. [DOI] [PubMed] [Google Scholar]


