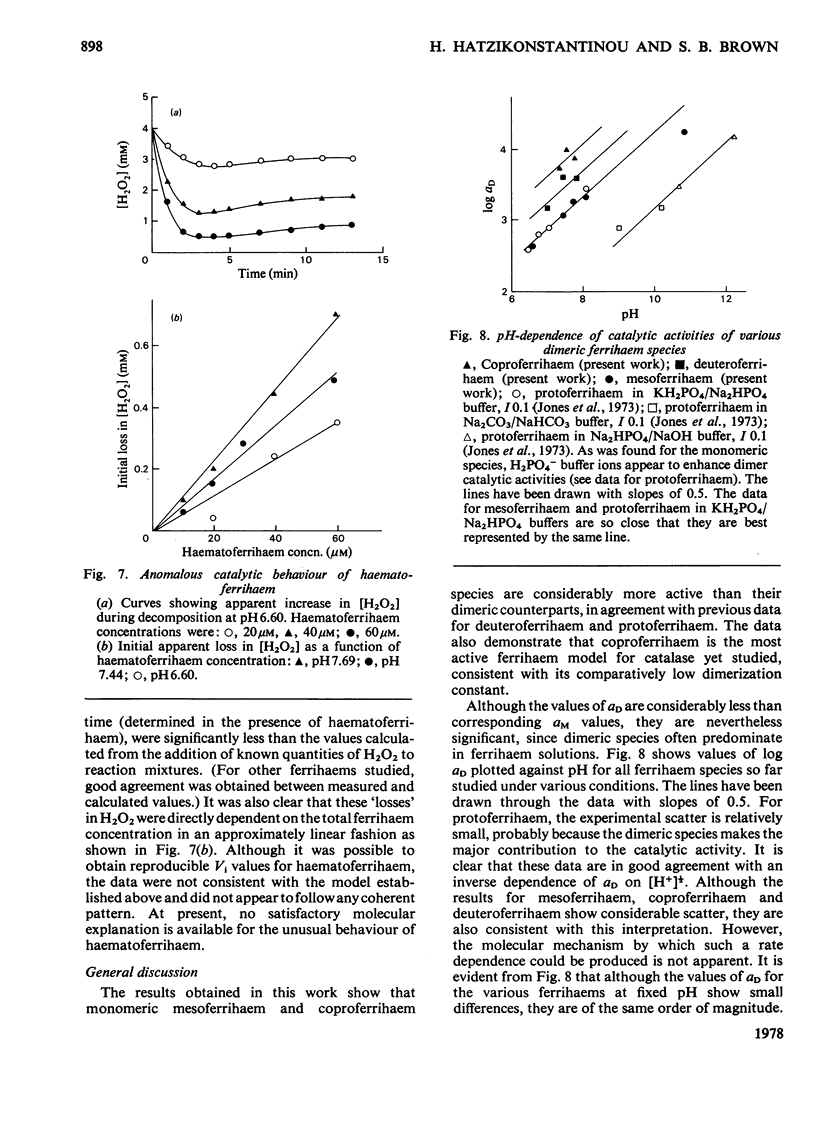
Abstract
The catalytic decomposition of H2O2 by deuteroferrihaem, mesoferrihaem, coproferrihaem and haematoferrihaem was studied as a model for the mechanism of action of catalase. For haematoferrihaem, anomalous but reproducible results were obtained, which could not be adequately explained. For each of the other ferrihaems studied, both monomeric and dimeric species catalysed decomposition, although the activity of monomer (aM) was much greater than that of dimer (aD). The pH variation of aD in the range 6.5--11 was consistent with an inverse dependence on [H+]1/2. The molecular mechanism whereby such a dependence could be achieved is not apparent. A study of the pH-dependence of aM in the range 6.5--11 revealed a linear inverse relationship with [H+]. This is interpreted in terms of attack by HO2- on ferrihaem monomer. The specific pH-independent rate constants for this reaction were in the order coproferrihaem greater than protoferrihaem greater than or equal to mesoferrihaem congruent to deuteroferrihaem. The order of magnitude of these rate constants is the same as that for catalysis by Fe(H2O)63+ and the second-order rate constant for decomposition of H2O2 by catalase. The implications on the mechanism of action of catalase are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blauer G., Zvilichovsky B. Ferriporphyrins in aqueous alkaline medium: the effects of salt and "ageing". Biochim Biophys Acta. 1970 Dec 22;221(3):442–449. doi: 10.1016/0005-2795(70)90215-1. [DOI] [PubMed] [Google Scholar]
- Brown S. B., Dean T. C., Jones P. Aggregation of ferrihaems. Dimerization and protolytic equilibria of protoferrihaem and deuteroferrihaem in aqueous solution. Biochem J. 1970 May;117(4):733–739. doi: 10.1042/bj1170733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. B., Dean T. C., Jones P. Catalatic activity of iron(3)-centred catalysts. Role of dimerization in the catalytic action of ferrihaems. Biochem J. 1970 May;117(4):741–744. doi: 10.1042/bj1170741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. B., Hatzikonstantinou H., Herries D. G. The dimerization of ferrihaems. I. The effect of buffer ions and specific cations on deuteroferrihaem dimerization. Biochim Biophys Acta. 1978 Mar 20;539(3):338–351. doi: 10.1016/0304-4165(78)90038-7. [DOI] [PubMed] [Google Scholar]
- Brown S. B., Hatzikonstantinou H. The dimerization of ferrihaems. II. Equilibrium and kinetic studies of mesoferrihaem dimerization. Biochim Biophys Acta. 1978 Mar 20;539(3):352–363. doi: 10.1016/0304-4165(78)90039-9. [DOI] [PubMed] [Google Scholar]
- Brown S. B., Jones P., Lantzke I. R. Infrared evidence for an oxo-bridged (Fe-O-Fe) haemin dimer. Nature. 1969 Aug 30;223(5209):960–961. doi: 10.1038/223960a0. [DOI] [PubMed] [Google Scholar]
- Jones P., Robson T., Brown S. B. The catalase activity of ferrihaems. Biochem J. 1973 Oct;135(2):353–359. doi: 10.1042/bj1350353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly H. C., Davies D. M., King M. J., Jones P. Pre-steady-state kinetics of intermediate formation in the deuteroferriheme-hydrogen peroxide system. Biochemistry. 1977 Aug 9;16(16):3543–3549. doi: 10.1021/bi00635a007. [DOI] [PubMed] [Google Scholar]


