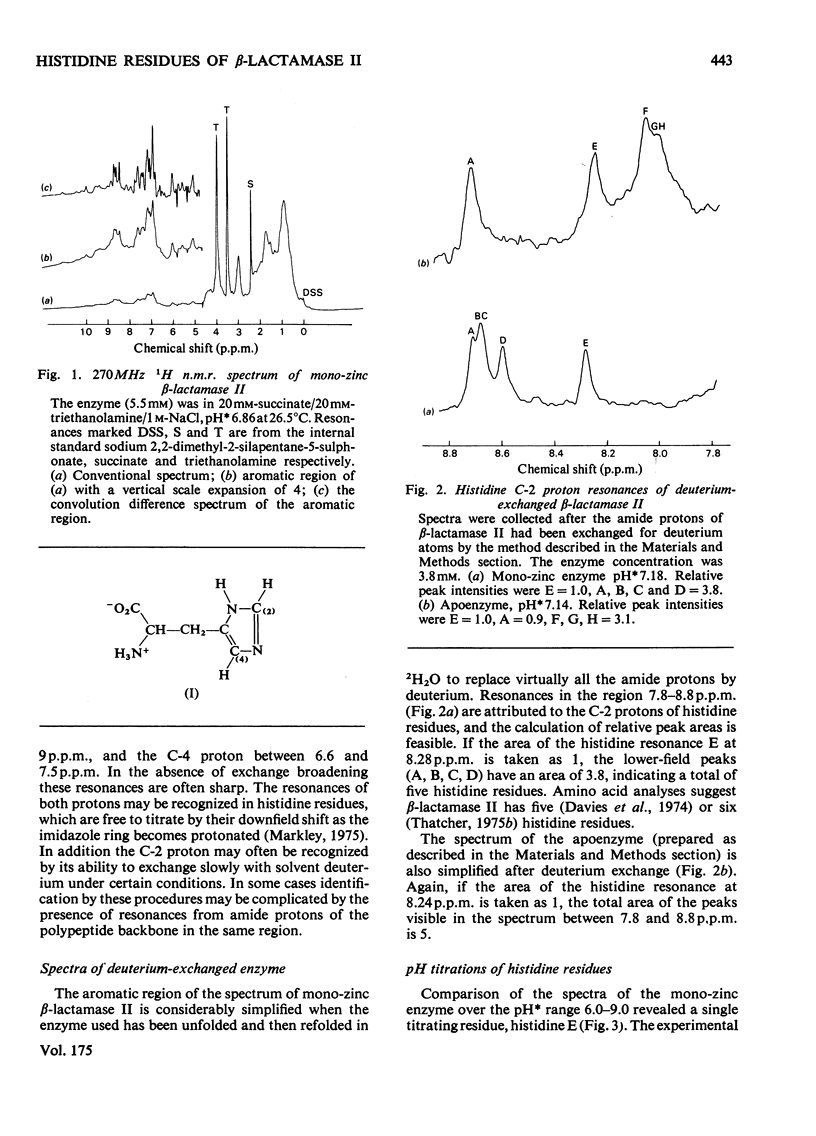
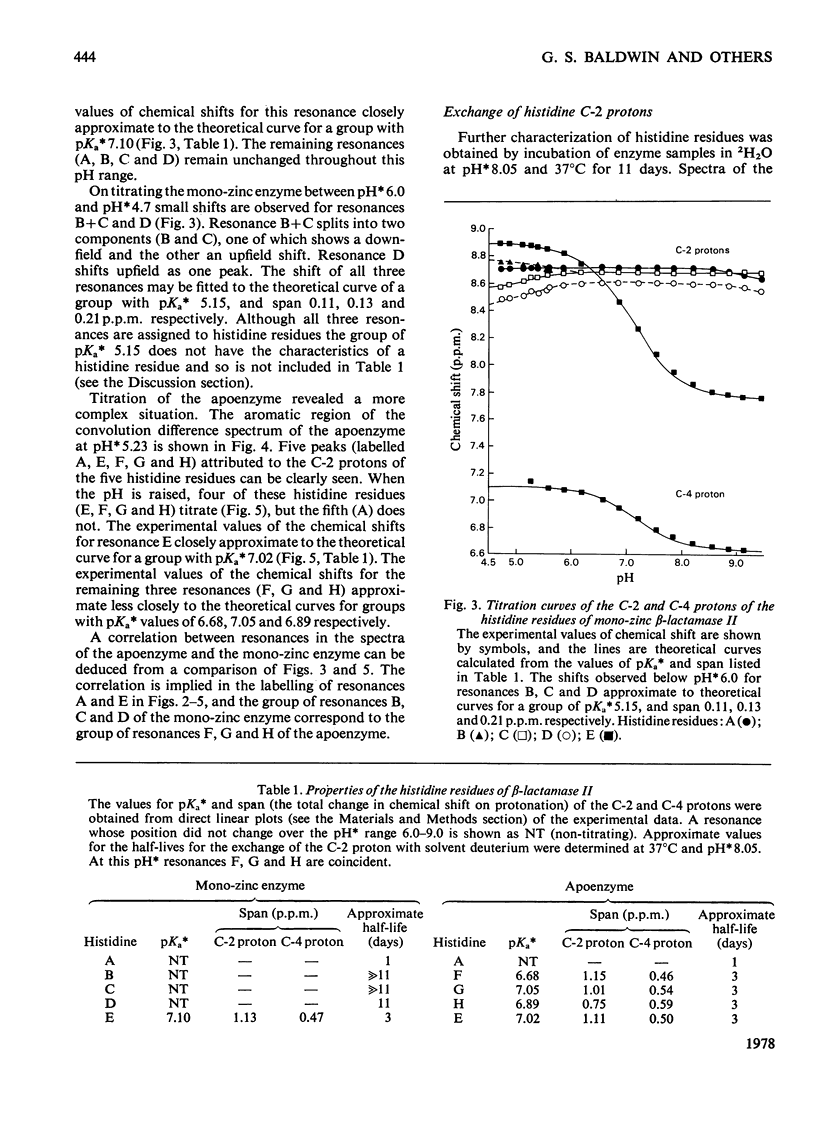
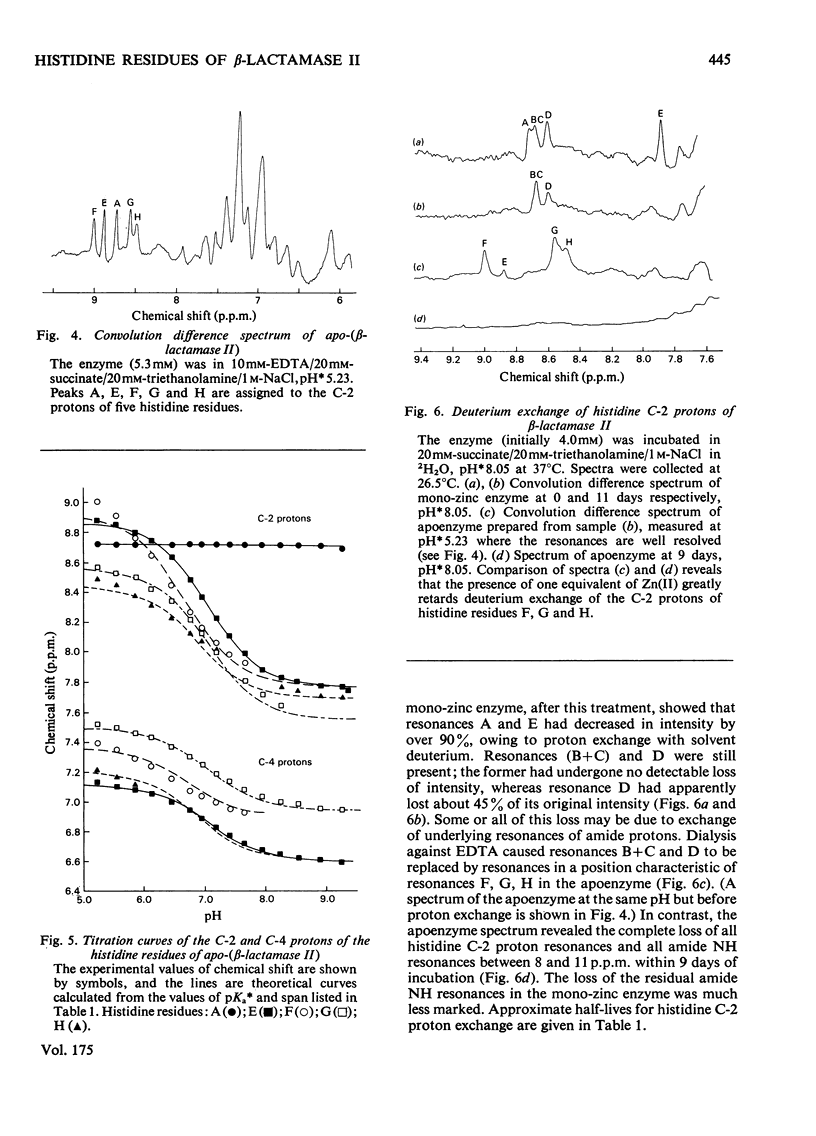
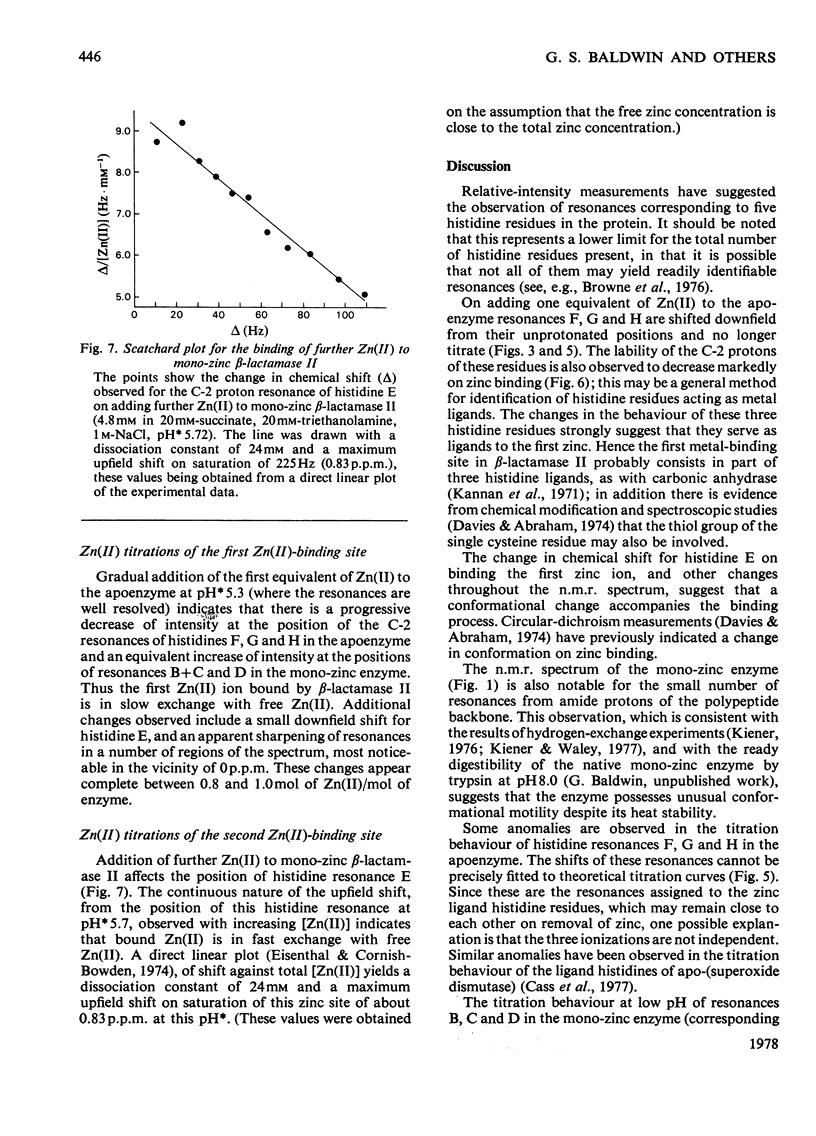
Abstract
1. The Zn(II)-requiring beta-lactamase from Bacillus cereus 569/H/9, which has two zinc-binding sites, was examined by 270 MHz 1H n.m.r. spectroscopy. Resonances were assigned to five histidine residues. 2. Resonances attributed to three of the histidine residues in the apoenzyme shift on the addition of one equivalent of Zn(II). 3. Although these three histidine residues are free to titrate in the apoenzyme, none of them titrates over the pH range 6.0--9.0 in the mono-zinc enzyme. 4. The ability of the C-2 protons of these three histidine residues to exchange with solvent (2H2O) is markedly decreased on Zn(II) binding. 5. It is proposed that these three histidine residues act as zinc ligands at the tighter zinc-binding site. 6. Resonances attributed to a fourth histidine residue shift on addition of further zinc to the mono-zinc enzyme. It is proposed that this histidine residue acts as a Zn(II) ligand at the second zinc-binding site.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler R. P., Meadway R. J. Chemical structure of bacterial penicillinases. Nature. 1969 Apr 5;222(5188):24–26. doi: 10.1038/222024a0. [DOI] [PubMed] [Google Scholar]
- Browne C. A., Campbell I. D., Kiener P. A., Phillips D. C., Waley S. G., Wilson I. A. Studies of the histidine residues of triose phosphate isomerase by proton magnetic resonance and x-ray crystallography. J Mol Biol. 1976 Jan 25;100(3):319–343. doi: 10.1016/s0022-2836(76)80066-6. [DOI] [PubMed] [Google Scholar]
- CROMPTON B., JAGO M., CRAWFORD K., NEWTON G. G., ABRAHAM E. P. Behaviour of some derivatives of 7-aminocephalosporanic acid and 6-aminopenicillanic acidas substrates, inhibitors and inducers of penicillinases. Biochem J. 1962 Apr;83:52–63. doi: 10.1042/bj0830052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell I. D., Lindskog S., White A. I. A study of the histidine residues of human carbonic anhydrase B using 270 MHz proton magnetic resonance. J Mol Biol. 1974 Dec 15;90(3):469–489. doi: 10.1016/0022-2836(74)90229-0. [DOI] [PubMed] [Google Scholar]
- Cass A. E., Hill A. O., Smith B. E., Bannister J. V., Bannister W. H. Investigation of the structure of bovine erythrocyte superoxide dismutase by 1H nuclear magnetic resonance spectroscopy. Biochemistry. 1977 Jul 12;16(14):3061–3066. doi: 10.1021/bi00633a003. [DOI] [PubMed] [Google Scholar]
- Davies R. B., Abraham E. P. Metal cofactor requirements of beta-lactamase II. Biochem J. 1974 Oct;143(1):129–135. doi: 10.1042/bj1430129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R. B., Abraham E. P. Separation, purification and properties of beta-lactamase I and beta-lactamase II from Bacillus cereus 569/H/9. Biochem J. 1974 Oct;143(1):115–127. doi: 10.1042/bj1430115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R. B. Comparison of beta-lactamase II from Bacillus cereus 569/H/9 with a beta-lactamase from Bacillus cereus 5/B/6. Biochem J. 1975 Feb;145(2):409–411. doi: 10.1042/bj1450409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworschack R. T., Plapp B. V. pH, isotope, and substituent effects on the interconversion of aromatic substrates catalyzed by hydroxybutyrimidylated liver alcohol dehydrogenase. Biochemistry. 1977 Jun 14;16(12):2716–2725. doi: 10.1021/bi00631a020. [DOI] [PubMed] [Google Scholar]
- Eisenthal R., Cornish-Bowden A. The direct linear plot. A new graphical procedure for estimating enzyme kinetic parameters. Biochem J. 1974 Jun;139(3):715–720. doi: 10.1042/bj1390715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris T. M. Deuterium exchange reactions at the 2-position of imidazoles. Chem Ind. 1965 Oct 9;41:1728–1729. [PubMed] [Google Scholar]
- Kannan K. K., Liljas A., Waara I., Bergstén P. C., Lövgren S., Strandberg B., Bengtsson U., Carlbom U., Fridborg K., Järup L. Crystal structure of human erythrocyte carbonic anhydrase C. VI. The three-dimensional structure at high resolution in relation to other mammalian carbonic anhydrases. Cold Spring Harb Symp Quant Biol. 1972;36:221–231. doi: 10.1101/sqb.1972.036.01.030. [DOI] [PubMed] [Google Scholar]
- Kannan K. K., Petef M., Fridborg K., Cid-Dresdner H., Lövgren S. Structure and function of carbonic anhydrases. Imidazole binding to human carbonic anhydrase B and the mechanism of action of carbonic anhydrases. FEBS Lett. 1977 Jan 15;73(1):115–119. doi: 10.1016/0014-5793(77)80027-6. [DOI] [PubMed] [Google Scholar]
- Kiener P. A., Waley S. G. Substrate-induced deactivation of penicillinases. Studies of beta-lactamase I by hydrogen exchange. Biochem J. 1977 Aug 1;165(2):279–285. doi: 10.1042/bj1650279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara S., Adams E. P., Abraham E. P. The composition of beta-lactamase I and beta-lactamase II from Bacillus cereus 569-H. Biochem J. 1970 Jul;118(3):475–480. doi: 10.1042/bj1180475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher D. R. Beta-lactamase (Bacillus cereus). Methods Enzymol. 1975;43:640–652. doi: 10.1016/0076-6879(75)43129-9. [DOI] [PubMed] [Google Scholar]
- Thatcher D. R. The partial amino acid sequence of the extracellular beta-lactamase I of Bacillus cereus 569/H. Biochem J. 1975 May;147(2):313–326. doi: 10.1042/bj1470313. [DOI] [PMC free article] [PubMed] [Google Scholar]


