Abstract
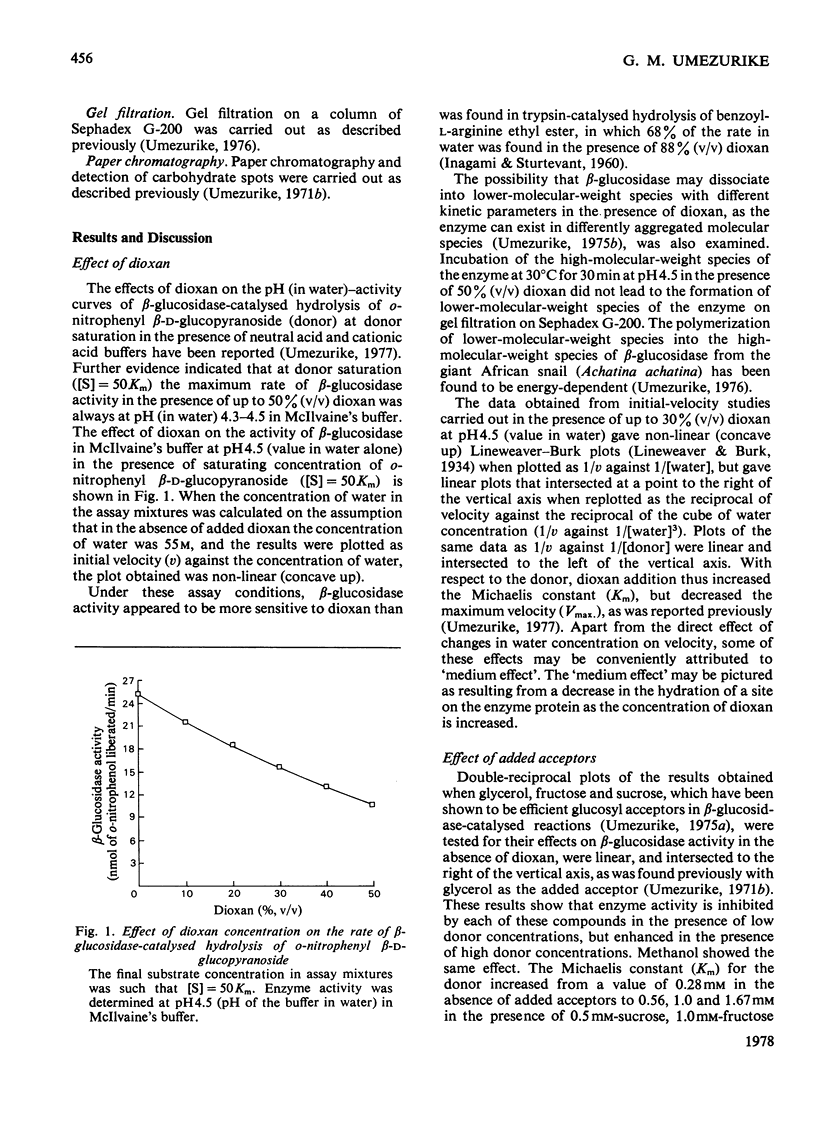
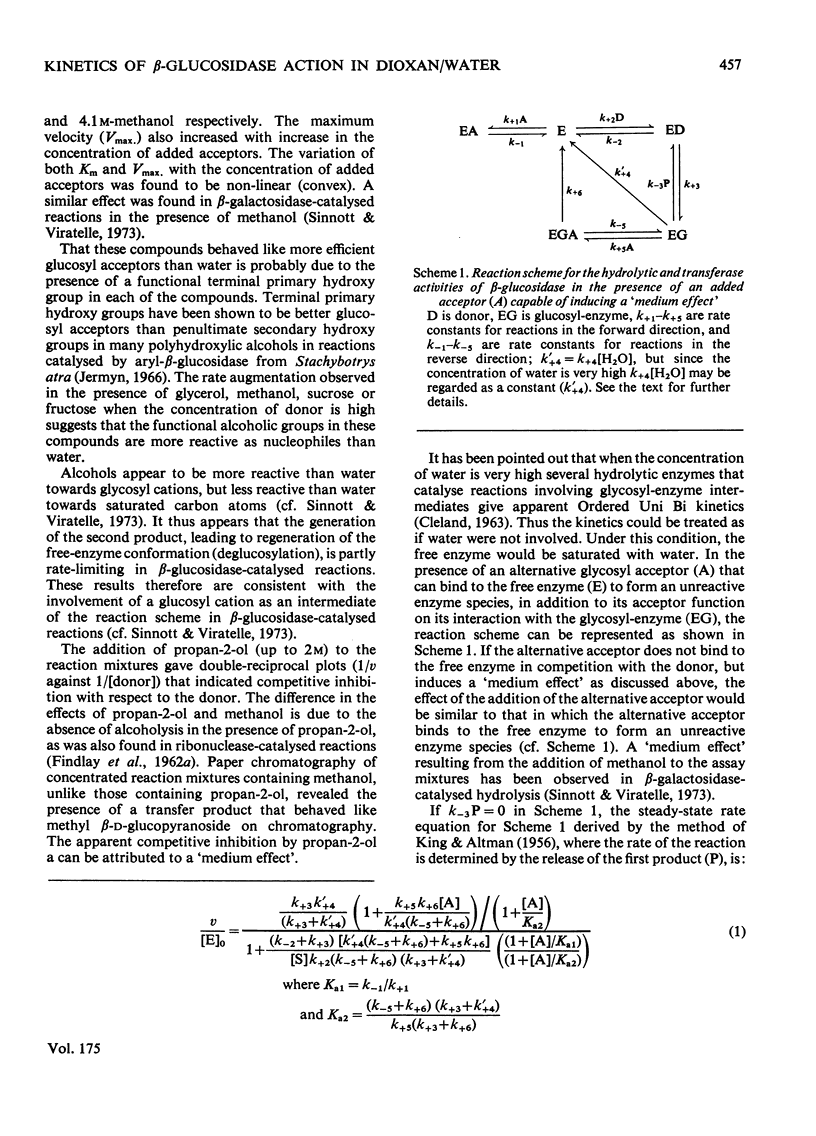
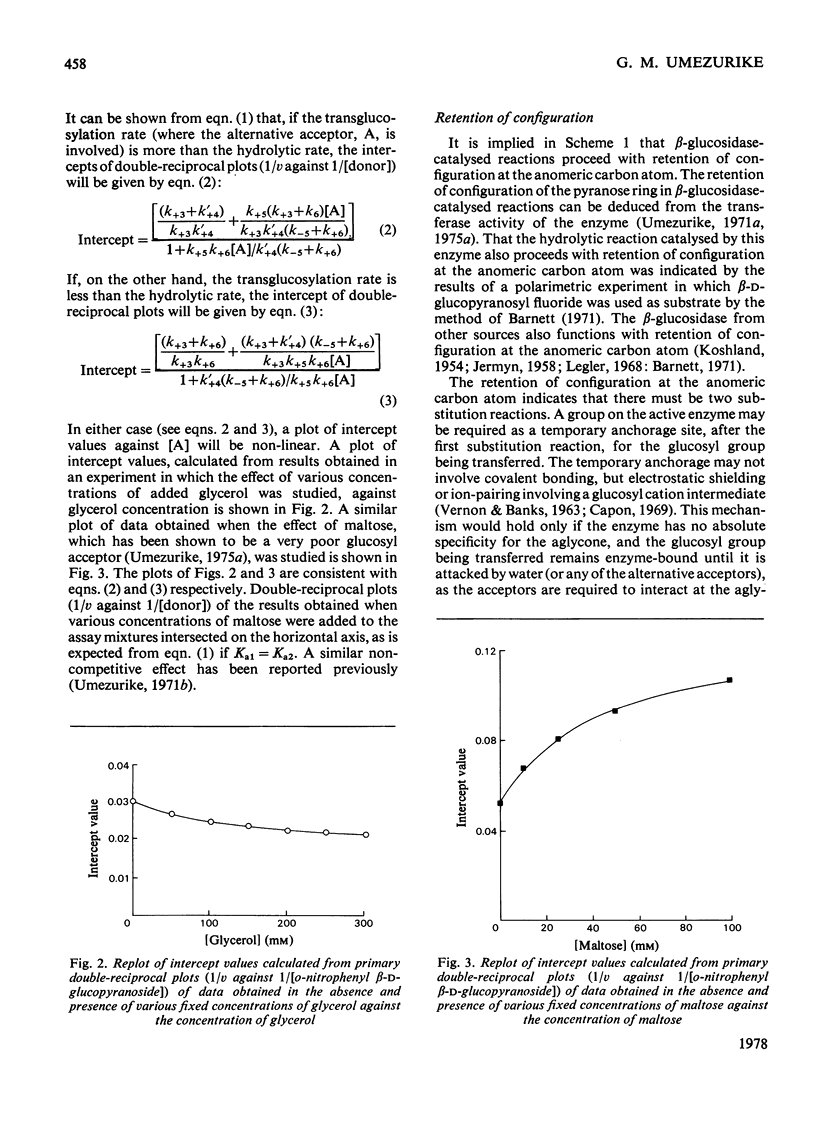
1. The hydrolysis of o-nitrophenyl beta-D-glucopyranoside by the high-molecular-weight beta-glucosidase (beta-D-glucoside glucohydrolase, EC 3.2.1.21) from Botryodiplodia theobromae Pat. has been studied in the presence of added dioxan. 2. At donor saturation, the maximum rate of hydrolysis in the presence of up to 50%(v/v) dioxan was pH4.3-4.5 (pH of the buffer system in water) in McIlvaine's buffer. 3. Increasing dioxan concentrations progressively decreased the maximum rate of hydrolysis. 4. The rate of enzyme-catalysed reaction was enhanced at high donor concentrations, but inhibited at low donor concentrations in the presence of glycerol, methanol, fructose of sucrose. 5. The hydrolytic reaction was found to proceed with retention of configuration at the anomeric carbon atom. 6. The kinetics of the enzyme-catalysed process in the presence of added acceptors indicated that water was necessary for the maintenance of the active enzyme conformation apart from its acceptor function.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett J. E. The hydrolysis of glycosyl fluorides by glycosidases. Determination of the anomeric configuration of the products of glycosidase action. Biochem J. 1971 Jul;123(4):607–611. doi: 10.1042/bj1230607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- Findlay D., Mathias A. P., Rabin B. R. The active site and mechanism of action of bovine pancreatic ribonuclease. 4. The activity in inert organic solvents and alcohols. Biochem J. 1962 Oct;85(1):134–139. doi: 10.1042/bj0850134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay D., Mathias A. P., Rabin B. R. The active site and mechanism of action of bovine pancreatic ribonuclease. 5. The charge types at the active centre. Biochem J. 1962 Oct;85(1):139–144. doi: 10.1042/bj0850139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INAGAMI T., STURTEVANT J. M. The trypsin-catalyzed hydrolysis of benzoyl-L-arginine ethyl ester. I. The kinetics in dioxane-water mixtures. Biochim Biophys Acta. 1960 Feb 12;38:64–79. doi: 10.1016/0006-3002(60)91196-3. [DOI] [PubMed] [Google Scholar]
- JERMYN M. A. Mechanism of carbohydrase action. Science. 1957 Jan 4;125(3236):12–15. doi: 10.1126/science.125.3236.12. [DOI] [PubMed] [Google Scholar]
- Legler G. Labelling of the active centre of a beta-glucosidase. Biochim Biophys Acta. 1968 Mar 25;151(3):728–729. doi: 10.1016/0005-2744(68)90033-8. [DOI] [PubMed] [Google Scholar]
- Sinnott M. L., Viratelle O. M. The effect of methanol and dioxan on the rates of the beta-galactosidase-catalysed hydrolyses of some beta-D-galactrophyranosides: rate-limiting degalactosylation. The ph-dependence of galactosylation and degalactosylation. Biochem J. 1973 May;133(1):81–87. doi: 10.1042/bj1330081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezurike G. M. Kinetic analysis of the mechanism of action of beta-glucosidase from Botryodiplodia theobromae Pat. Biochim Biophys Acta. 1975 Jul 27;397(1):164–178. doi: 10.1016/0005-2744(75)90190-4. [DOI] [PubMed] [Google Scholar]
- Umezurike G. M. Kinetic properties of -glucosidase from Botryodiplodia theobromae Pat. Biochim Biophys Acta. 1971 Oct;250(1):182–191. doi: 10.1016/0005-2744(71)90132-x. [DOI] [PubMed] [Google Scholar]
- Umezurike G. M. The active site of beta-glucosidase from Botryodiplodia theobromae. Effects of pH and dioxan on enzyme-catalysed reactions. Biochem J. 1977 Dec 1;167(3):831–833. doi: 10.1042/bj1670831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezurike G. M. The beta-glucosidase in the gut contents of the snail Achatina achatina. Biochem J. 1976 Aug 1;157(2):381–387. doi: 10.1042/bj1570381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezurike G. M. The purification and properties of extracellular beta-glucosidase from Botryodiplodia theobromae Pat. Biochim Biophys Acta. 1971 Feb 10;227(2):419–428. doi: 10.1016/0005-2744(71)90073-8. [DOI] [PubMed] [Google Scholar]
- Umezurike G. M. The subunit structure of beta-glucosidase from Botryodiplodia theobromae Pat. Biochem J. 1975 Feb;145(2):361–368. doi: 10.1042/bj1450361. [DOI] [PMC free article] [PubMed] [Google Scholar]


