Abstract
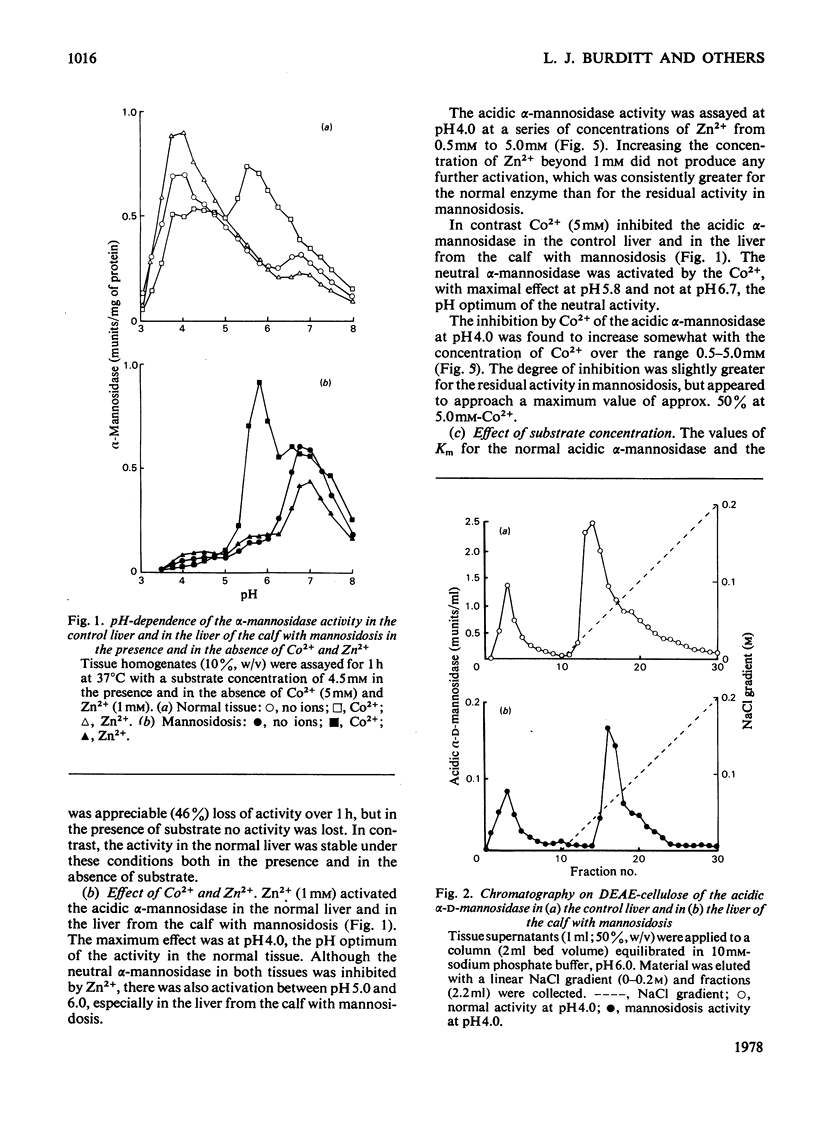
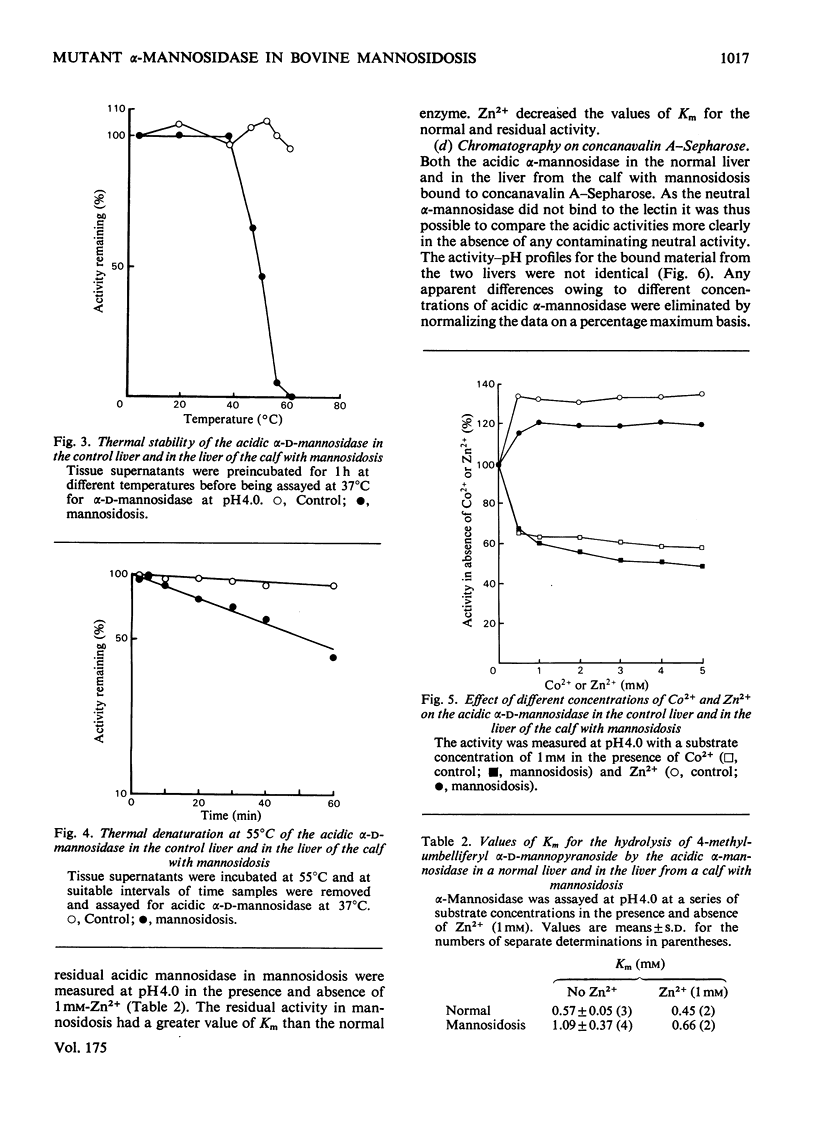
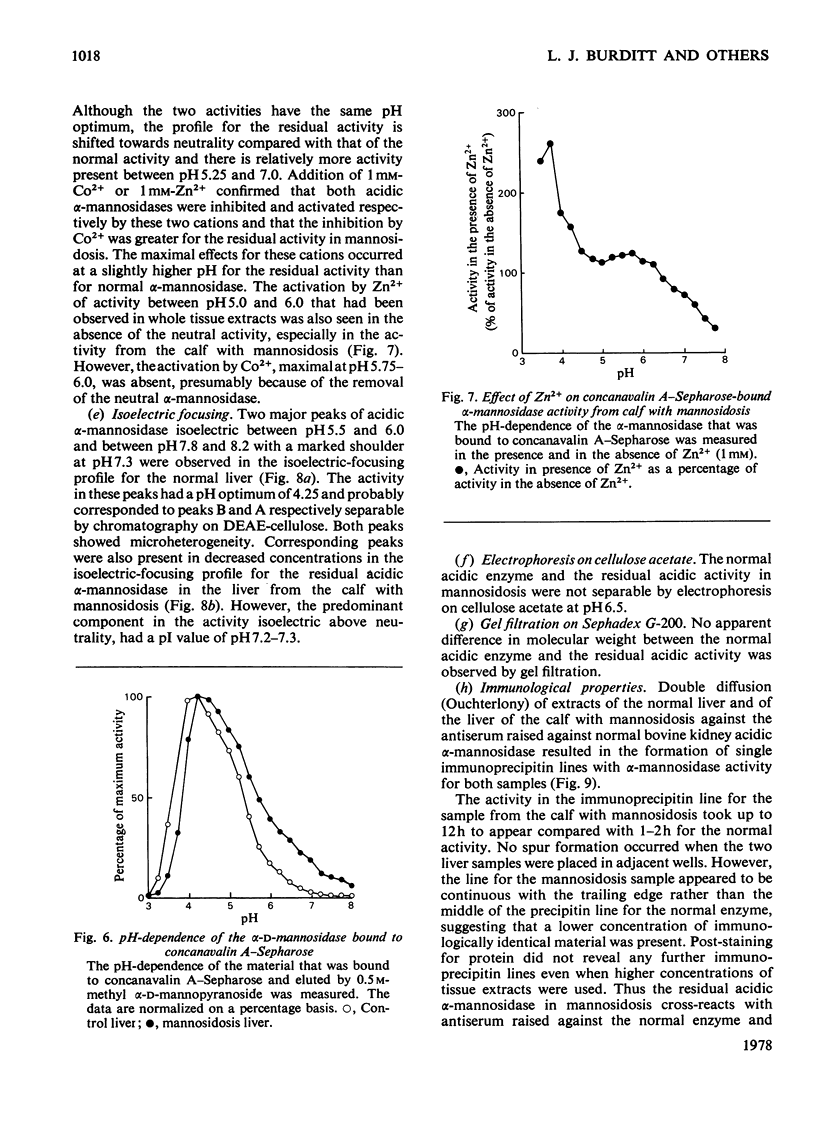
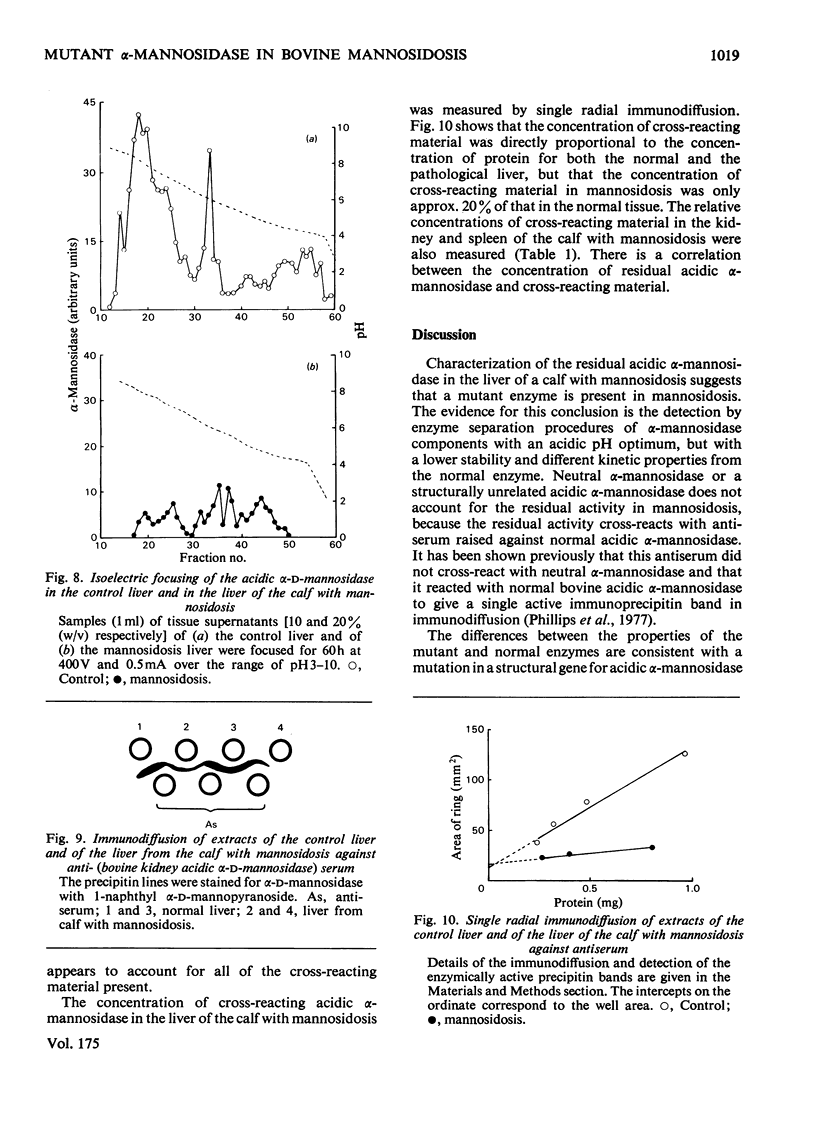
Residual acidic α-mannosidase, varying in amount up to approx. 15% of normal values, can be measured in various organs of a calf with mannosidosis. The highest specific activity and relative proportion of residual activity were found in the liver. Chromatography on DEAE-cellulose showed that the residual activity was associated with two components, which were eluted at comparable positions with those found in normal tissues. The residual activity had a lower thermal stability and a higher Km value for a synthetic substrate than did the normal enzyme. No differences in molecular weight or electrophoretic mobility between normal acidic α-mannosidase and the residual activity were observed by gel filtration and electrophoresis on cellulose acetate respectively. The isoelectric focusing profiles for the α-mannosidase in the normal and pathological livers were very similar. It is suggested that a mutant enzyme, resulting from a mutation in a structural gene, accounts for the residual acidic α-mannosidase in mannosidosis. The mutant enzyme, which cross-reacts with antiserum raised against normal bovine acidic α-mannosidase, is present at a decreased concentration compared with the normal enzyme. There is a correlation between the concentrations of residual activity and cross-reacting material in mannosidosis. α-Mannosidase with a pH optimum of 5.75 and which is activated by Zn2+ was also detected in the liver of the calf with mannosidosis. However, it is probably not a product of the defective gene because addition of Zn2+ indicated that it was also present in normal tissues.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaudet A. L., Nichols B. L., Jr Residual altered alpha-mannosidase in human mannosidosis. Biochem Biophys Res Commun. 1976 Jan 12;68(1):292–298. doi: 10.1016/0006-291x(76)90042-5. [DOI] [PubMed] [Google Scholar]
- Burditt L. J., Winchester B. G., Van-de-Water N. S., Jolly R. D. Preliminary characterization of the mutant alpha-mannosidase in bovine mannosidosis [proceedings]. Biochem Soc Trans. 1978;6(2):438–440. doi: 10.1042/bst0060438. [DOI] [PubMed] [Google Scholar]
- Desnick R. J., Sharp H. L., Grabowski G. A., Brunning R. D., Quie P. G., Sung J. H., Gorlin R. J., Ikonne J. U. Mannosidosis: clinical, morphologic, immunologic, and biochemical studies. Pediatr Res. 1976 Dec;10(12):985–996. doi: 10.1203/00006450-197612000-00008. [DOI] [PubMed] [Google Scholar]
- Desnick R. J., Thorpe S. R., Fiddler M. B. Toward enzyme therapy for lysosomal storage diseases. Physiol Rev. 1976 Jan;56(1):57–99. doi: 10.1152/physrev.1976.56.1.57. [DOI] [PubMed] [Google Scholar]
- Fluharty A. L., Lassila E. L., Porter M. T., Kihara H. The electrophoretic separation of human -galactosidases on cellulose acetate. Biochem Med. 1971 Apr;5(2):158–164. doi: 10.1016/0006-2944(71)90083-4. [DOI] [PubMed] [Google Scholar]
- Hocking J. D., Jolly R. D., Batt R. D. Deficiency of alpha-mannosidase in Angus cattle. An inherited lysosomal storage disease. Biochem J. 1972 Jun;128(1):69–78. doi: 10.1042/bj1280069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultberg B., Masson P. K. Activation of residual acidic alpha-mannosidase activity in mannosidosis tissues by metal ions. Biochem Biophys Res Commun. 1975 Dec 15;67(4):1473–1479. doi: 10.1016/0006-291x(75)90192-8. [DOI] [PubMed] [Google Scholar]
- Hultberg B. Properties of alpha-mannosidase in mannosidosis. Scand J Clin Lab Invest. 1970 Sep;26(2):155–159. doi: 10.3109/00365517009049228. [DOI] [PubMed] [Google Scholar]
- Jolly R. D. Mannosidosis of Angus Cattle: a prototype control program for some genetic diseases. Adv Vet Sci Comp Med. 1975;19:1–21. [PubMed] [Google Scholar]
- Jolly R. D. The pathology of the central nervous system in pseudolipidosis of Angus calves. J Pathol. 1971 Feb;103(2):113–121. doi: 10.1002/path.1711030206. [DOI] [PubMed] [Google Scholar]
- Jolly R. D., Thompson K. G., Murphy C. E., Manktelow B. W., Bruere A. N., Winchester B. G. Enzyme replacement therapy--an experiment of nature in a chimeric mannosidosis calf. Pediatr Res. 1976 Apr;10(4):219–224. doi: 10.1203/00006450-197604000-00003. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Marinkovic D. V., Marinkovic J. N. Isolation and properties of alpha-D-mannosidase from human kidney. Biochem J. 1976 May 1;155(2):217–223. doi: 10.1042/bj1550217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mersmann G., Buddecke E. Evidence for material from mannosidosis fibroblasts crossreacting with anti-acidic alpha-mannosidase antibodies. FEBS Lett. 1977 Jan 15;73(1):123–126. [PubMed] [Google Scholar]
- Phillips N. C., Robinson D., Winchester B. G. Characterization of human liver alpha-D-mannosidase purified by affinity chromatography. Biochem J. 1976 Mar 1;153(3):579–587. doi: 10.1042/bj1530579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips N. C., Robinson D., Winchester B. G., Jolly R. D. Mannosidosis in Angus cattle. The enzymic defect. Biochem J. 1974 Feb;137(2):363–371. doi: 10.1042/bj1370363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips N. C., Winchester B. G. A serological investigation into the acidic alpha-D-mannosidase in normal Angus cattle and in a calf with mannosidosis. Biochem J. 1977 May 1;163(2):269–277. doi: 10.1042/bj1630269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips N., Robinson D., Winchester B. Immunological characterization of human liver alpha-D-mannosidase. Biochem J. 1975 Dec;151(3):469–475. doi: 10.1042/bj1510469a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaith S. M. Multiple alpha-mannosidase activities in mammalian tissues as shown by metal-ion activation. Biochem J. 1977 Jun 1;163(3):557–564. doi: 10.1042/bj1630557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulsiani D. R., Opheim D. J., Touster O. Purification and characterization of alpha-D-mannosidase from rat liver golgi membranes. J Biol Chem. 1977 May 25;252(10):3227–3233. [PubMed] [Google Scholar]
- Winchester B. G., Van-de-Water N. S., Jolly R. D. The nature of the residual alpha-mannosidase in plasma in bovine mannosidosis. Biochem J. 1976 Jul 1;157(1):183–188. doi: 10.1042/bj1570183. [DOI] [PMC free article] [PubMed] [Google Scholar]


