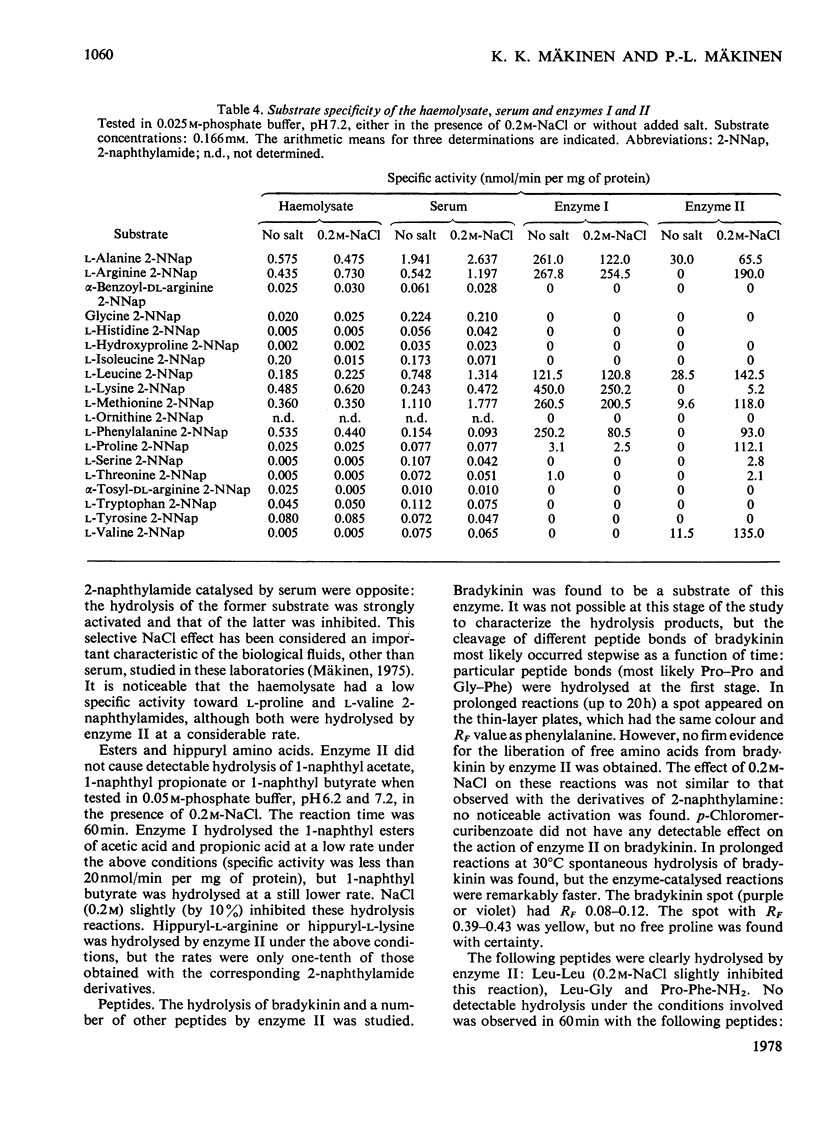
Abstract
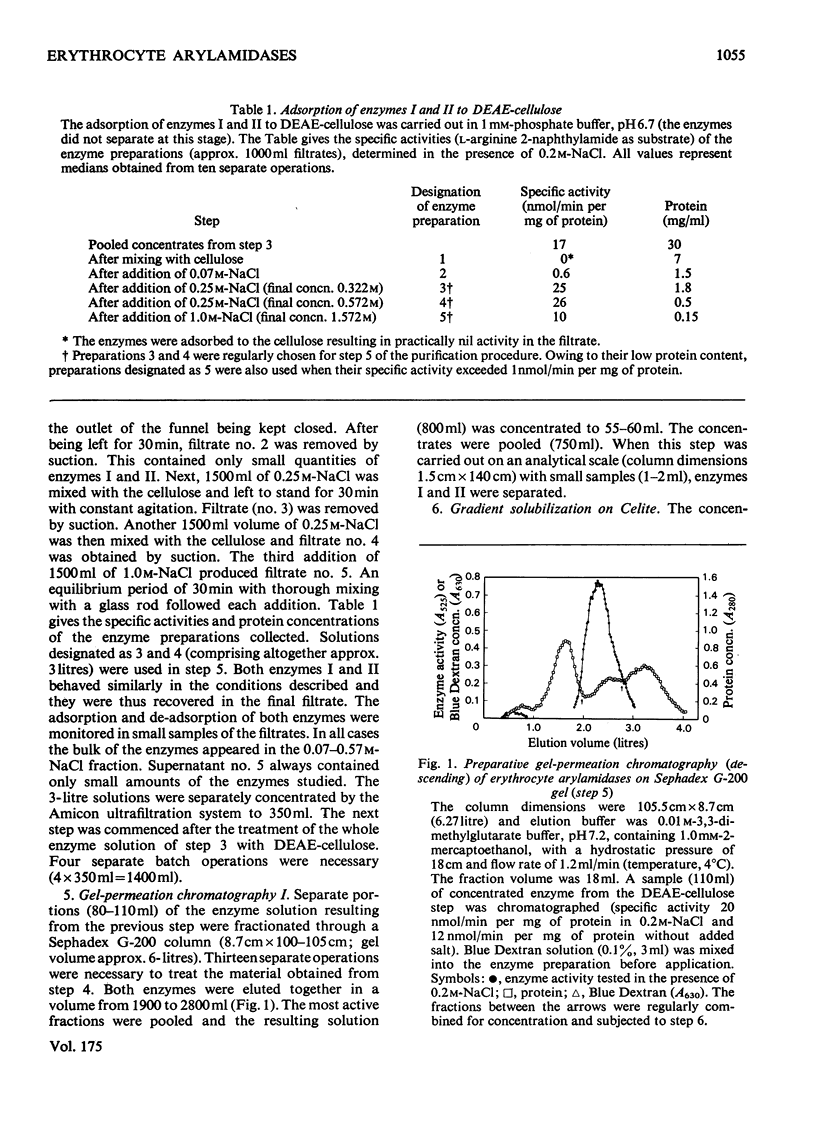
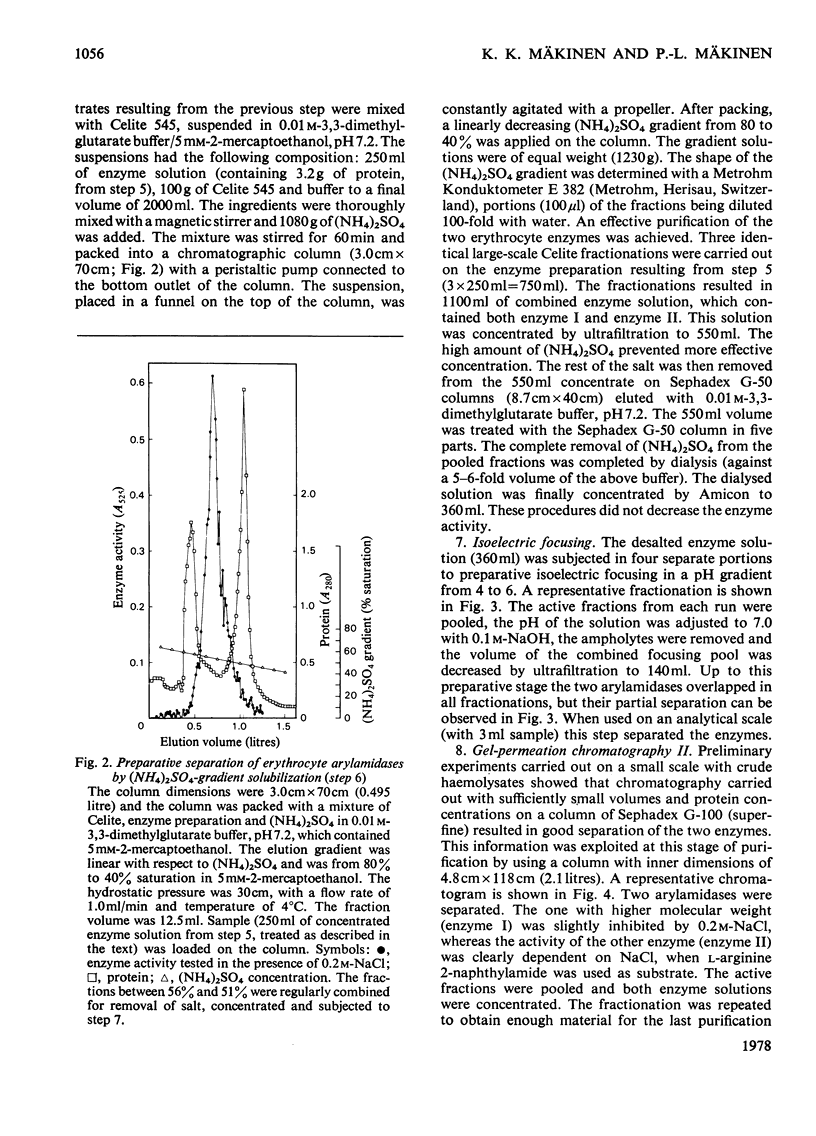
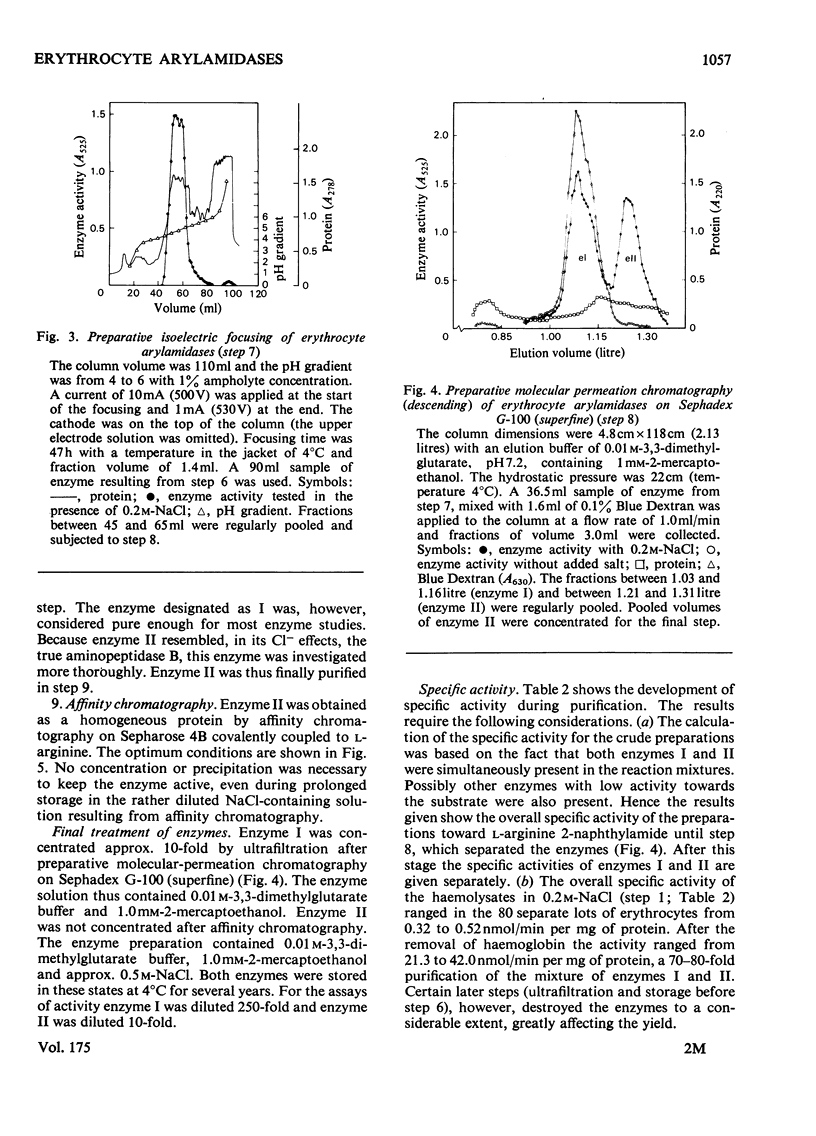
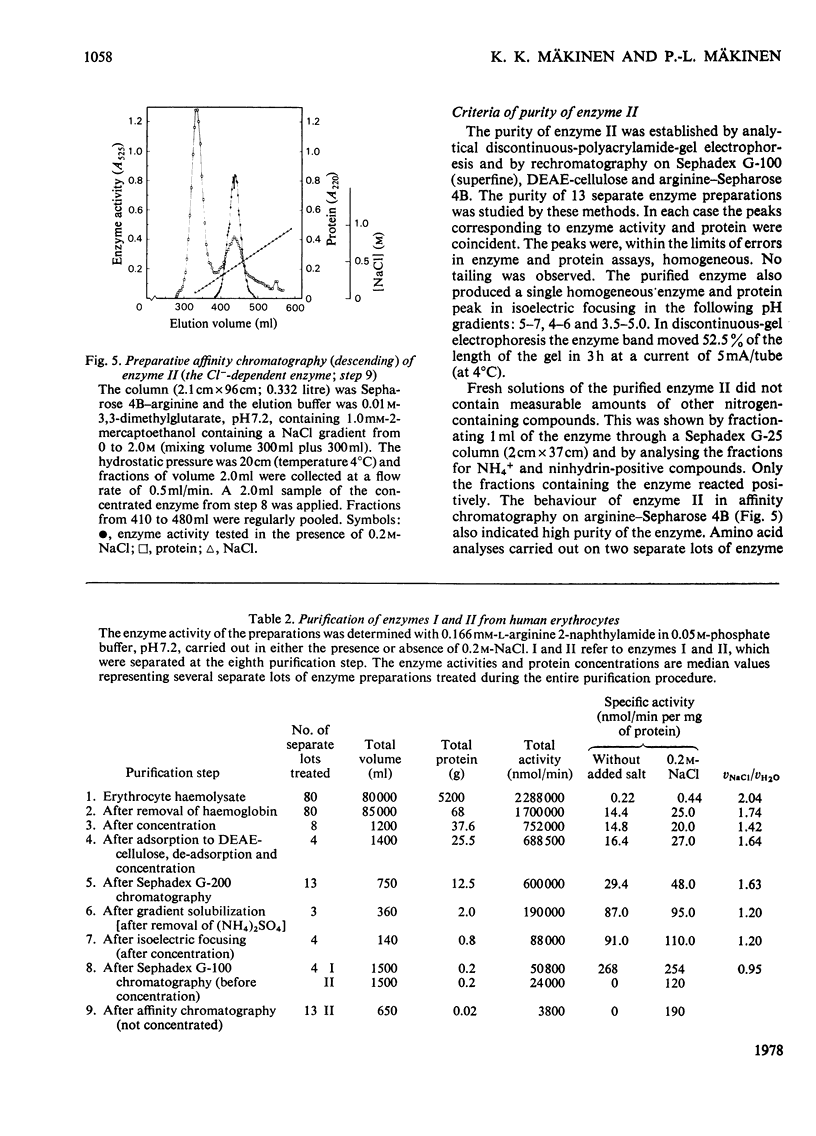
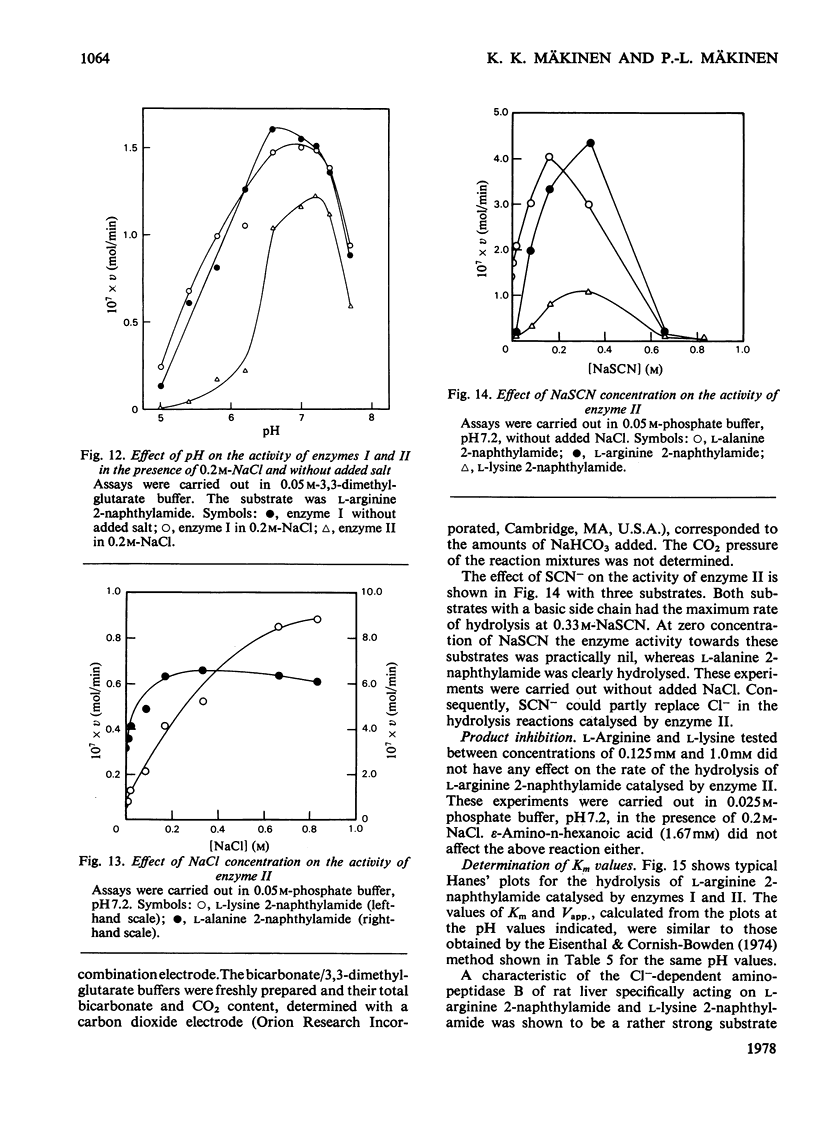
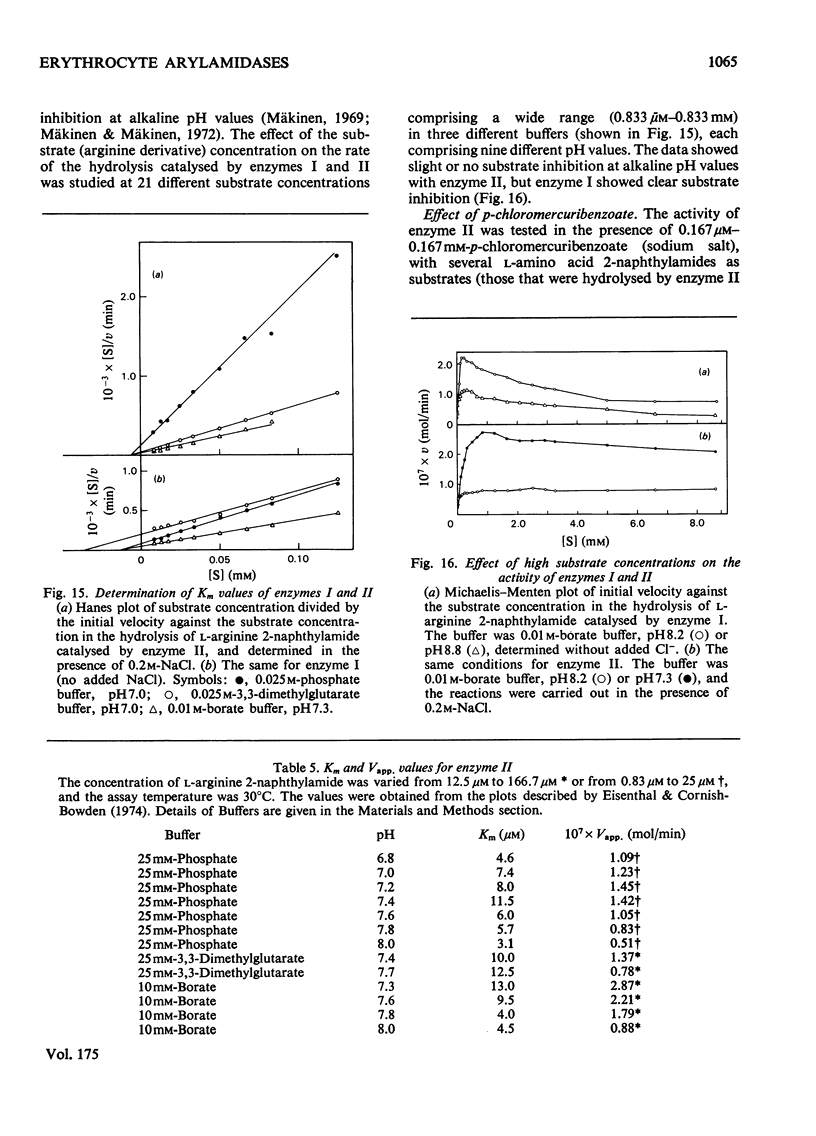
Two arylamidases (I and II) were purified from human erythrocytes by a procedure that comprised removal of haemoglobin from disrupted cells with CM-Sephadex D-50, followed by treatment of the haemoglobin-free preparation subsequently with DEAE-cellulose, gel-permeation chromatography on Sephadex G-200, gradient solubilization on Celite, isoelectric focusing in a pH gradient from 4 to 6, gel-permeation chromatography on Sephadex G-100 (superfine), and finally affinity chromatography on Sepharose 4B covalently coupled to L-arginine. In preparative-scale purifications, enzymes I and II were separated at the second gel-permeation chromatography. Enzyme II was obtained as a homogeneous protein, as shown by several criteria. Enzyme I hydrolysed, with decreasing rates, the L-amino acid 2-naphtylamides of lysine, arginine, alanine, methionine, phenylalanine and leucine, and the reactions were slightly inhibited by 0.2 M-NaCl. Enzyme II hydrolysed most rapidly the corresponding derivatives of arginine, leucine, valine, methionine, proline and alanine, in that order, and the hydrolyses were strongly dependent on Cl-. The hydrolysis of these substrates proceeded rapidly at physiological Cl- concentration (0.15 M). The molecular weights (by gel filtration) of enzymes I and II were 85 000 and 52 500 respectively. The pH optimum was approx. 7.2 for both enzymes. The isoelectric point of enzyme II was approx. 4.8. Enzyme I was activated by Co2+, which did not affect enzyme II to any noticeable extent. The kinetics of reactions catalysed by enzyme I were characterized by strong substrate inhibition, but enzyme II was not inhibited by high substrate concentrations. The Cl- activated enzyme II also showed endopeptidase activity in hydrolysing bradykinin.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bury A. F., Coolbear T., Savery C. R. Separation and properties of two arylamidases from rat cardiac-muscle extracts. Biochem J. 1977 Jun 1;163(3):565–570. doi: 10.1042/bj1630565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Eisenthal R., Cornish-Bowden A. The direct linear plot. A new graphical procedure for estimating enzyme kinetic parameters. Biochem J. 1974 Jun;139(3):715–720. doi: 10.1042/bj1390715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner C. W., Behal F. J. Hydrophobic binding sites of human liver alanine aminopeptidase. Arch Biochem Biophys. 1977 Aug;182(2):667–673. doi: 10.1016/0003-9861(77)90547-1. [DOI] [PubMed] [Google Scholar]
- Greiff D., Brooker D., Mackey S. Cryobiology of platelets. I. Aminopeptidase activity as a measure of intactness of platelets in vitro. Cryobiology. 1969 Nov-Dec;6(3):194–199. doi: 10.1016/s0011-2240(69)80349-4. [DOI] [PubMed] [Google Scholar]
- Hopsu V. K., Mäkinen K. K., Glenner G. G. Purification of a mammalian peptidase selective for N-terminal arginine and lysine residues: aminopeptidase B. Arch Biochem Biophys. 1966 Jan;114(3):557–566. doi: 10.1016/0003-9861(66)90380-8. [DOI] [PubMed] [Google Scholar]
- Hopsu V. K., Mäkinn K. K., Glenner G. G. Characterization of aminopeptidase B: substrate specificity and affector studies. Arch Biochem Biophys. 1966 Jun;114(3):567–575. doi: 10.1016/0003-9861(66)90381-x. [DOI] [PubMed] [Google Scholar]
- King T. P. Separation of proteins by ammonium sulfate gradient solubilization. Biochemistry. 1972 Feb 1;11(3):367–371. doi: 10.1021/bi00753a010. [DOI] [PubMed] [Google Scholar]
- Klimek R. Clinical studies on the balance between isooxytocinases in the blood of pregnant women. Clin Chim Acta. 1968 May;20(2):233–238. doi: 10.1016/0009-8981(68)90155-1. [DOI] [PubMed] [Google Scholar]
- Klimek R., Malolepszy E. Blood levels of cystine aminopeptidase (oxytocinase) in patients with toxic and simple goiter. Clin Chim Acta. 1968 Dec;22(4):491–495. doi: 10.1016/0009-8981(68)90097-1. [DOI] [PubMed] [Google Scholar]
- Knuuttila M., Virtanen K., Söderling E., Mäkinen K. K. A chloride-activated aminopeptidase in rat inflammatory exudate: properties and evidence of the origin of the enzyme. Biochem Biophys Res Commun. 1978 Mar 30;81(2):374–381. doi: 10.1016/0006-291x(78)91543-7. [DOI] [PubMed] [Google Scholar]
- Kurtz A. B., Wachsmuth E. D. Identification of plasma angiotensinase as aminopeptidase. Nature. 1969 Jan 4;221(5175):92–93. doi: 10.1038/221092a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mäkinen K. K., Brummer R., Scheinin A. Arylaminopeptidase activity in dental pulp. Acta Odontol Scand. 1970 Jun;28(3):377–387. doi: 10.3109/00016357009032041. [DOI] [PubMed] [Google Scholar]
- Mäkinen K. K. Evidence for the aggregation of aminopeptidase B during storage and breakdown of the aggregate by substrate and serum albumin. Biochim Biophys Acta. 1972 Jul 21;271(2):413–418. doi: 10.1016/0005-2795(72)90216-4. [DOI] [PubMed] [Google Scholar]
- Mäkinen K. K., Hopsu-Havu V. K. The presence of enzymes resembling aminopeptidase B in several rat organs. Ann Med Exp Biol Fenn. 1967;45(2):230–234. [PubMed] [Google Scholar]
- Mäkinen K. K., Hyyppä T. A biochemical study of the origin of arginine aminopeptidases in human gingival fluid. Arch Oral Biol. 1975 Aug;20(8):509–514. doi: 10.1016/0003-9969(75)90213-7. [DOI] [PubMed] [Google Scholar]
- Mäkinen K. K., Luostarinen V., Varrela J., Rekola M., Luoma S. Arginine aminopeptidase reactions to laser in vivo and in vitro. Biochem Med. 1975 Jun;13(2):192–195. doi: 10.1016/0006-2944(75)90155-6. [DOI] [PubMed] [Google Scholar]
- Mäkinen K. K., Oksala E. Evidence on the involvement in inflammation of an enzyme resembling aminopeptidase B. Clin Chim Acta. 1973 Dec 27;49(3):301–309. doi: 10.1016/0009-8981(73)90226-x. [DOI] [PubMed] [Google Scholar]
- Mäkinen K. K., Paunio K. U. A histochemical method for the demonstration of aminopeptidase B activity. J Histochem Cytochem. 1972 Mar;20(3):192–194. doi: 10.1177/20.3.192. [DOI] [PubMed] [Google Scholar]
- Mäkinen K. K., Virtanen K. K. Aminopeptidase B in human serum. Clin Chim Acta. 1976 Mar 1;67(2):213–218. doi: 10.1016/0009-8981(76)90262-x. [DOI] [PubMed] [Google Scholar]
- Mäkinen K. K., Virtanen K. K. Aminopeptidases of mechanically strained and normal rat gingiva, with special reference to aminopeptidase B. Acta Odontol Scand. 1974;32(2):115–124. doi: 10.3109/00016357409002540. [DOI] [PubMed] [Google Scholar]
- Mäkinen P. L., Mäkinen K. K. Fractionation and properties of aminopeptidase B during purification and storage. Int J Pept Protein Res. 1972;4(4):241–255. doi: 10.1111/j.1399-3011.1972.tb03425.x. [DOI] [PubMed] [Google Scholar]
- Nagatsu I., Nagatsu T., Yamamoto T., Glenner G. G., Mehl J. W. Purification of aminopeptidase A in human serum and degradation of angiotensin II by the purified enzyme. Biochim Biophys Acta. 1970 Feb 11;198(2):255–270. doi: 10.1016/0005-2744(70)90058-6. [DOI] [PubMed] [Google Scholar]
- Neef L., Peters J. E., Haschen R. J. Alanin- und Leuzinaminopeptidase in isolierten menschlichen Blutzellen. Z Gesamte Inn Med. 1973 Oct 1;28(19):573–576. [PubMed] [Google Scholar]
- SEARCY R. L., GOUGH G. S., KOROTZER J. L., BERGQUIST L. M. Evaluation of a new technique for estimation of urea nitrogen in serum. Am J Med Technol. 1961 Sep-Oct;27:255–262. [PubMed] [Google Scholar]
- Virtanen K. K., Mäkinen K. K., Oksala E. Activity of arginine aminopeptidases and phosphatases in inflamed palatal mucosa in denture stomatitis: a histochemical and biochemical study. J Dent Res. 1977 Jun;56(6):674–684. doi: 10.1177/00220345770560061801. [DOI] [PubMed] [Google Scholar]
- Yman L. Studies on human serum aminopeptidases. Some properties of oxytocinase, human serum aminopeptidase A and leucine aminopeptidase and their purification from retroplacental serum. Acta Pharm Suec. 1970 Apr;7(2):75–86. [PubMed] [Google Scholar]


