Abstract
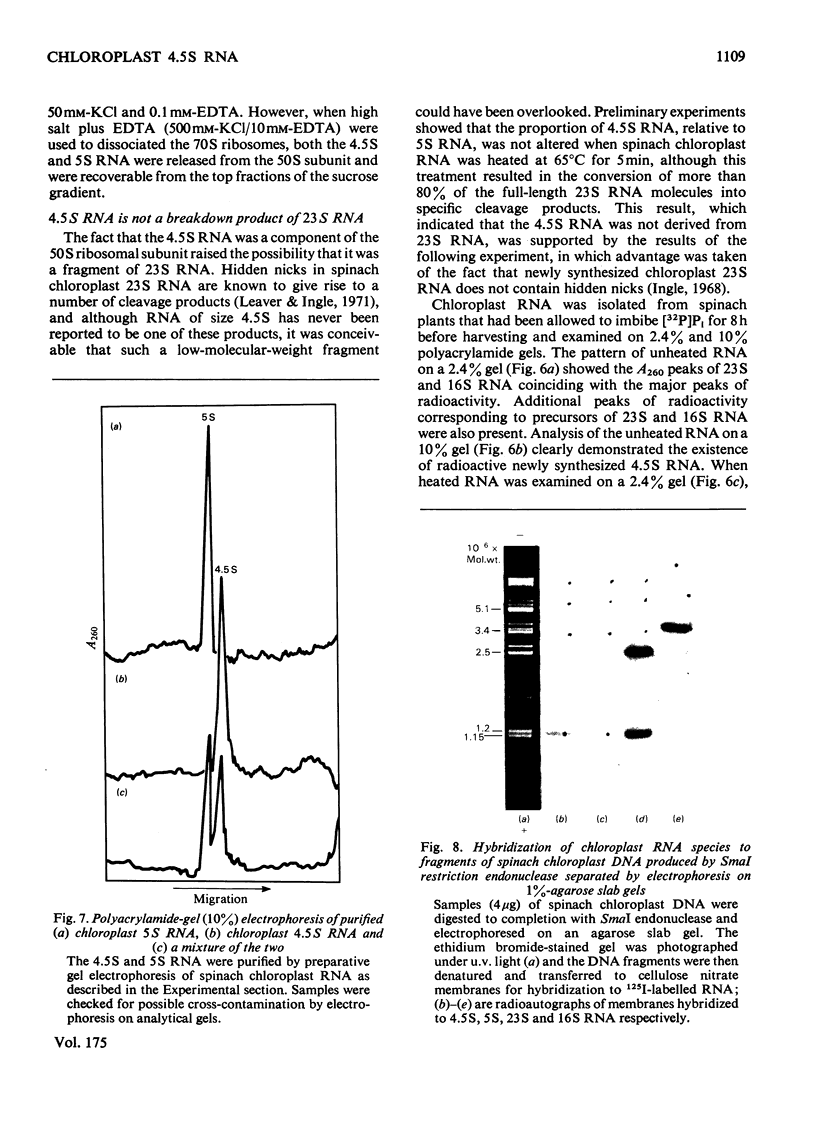
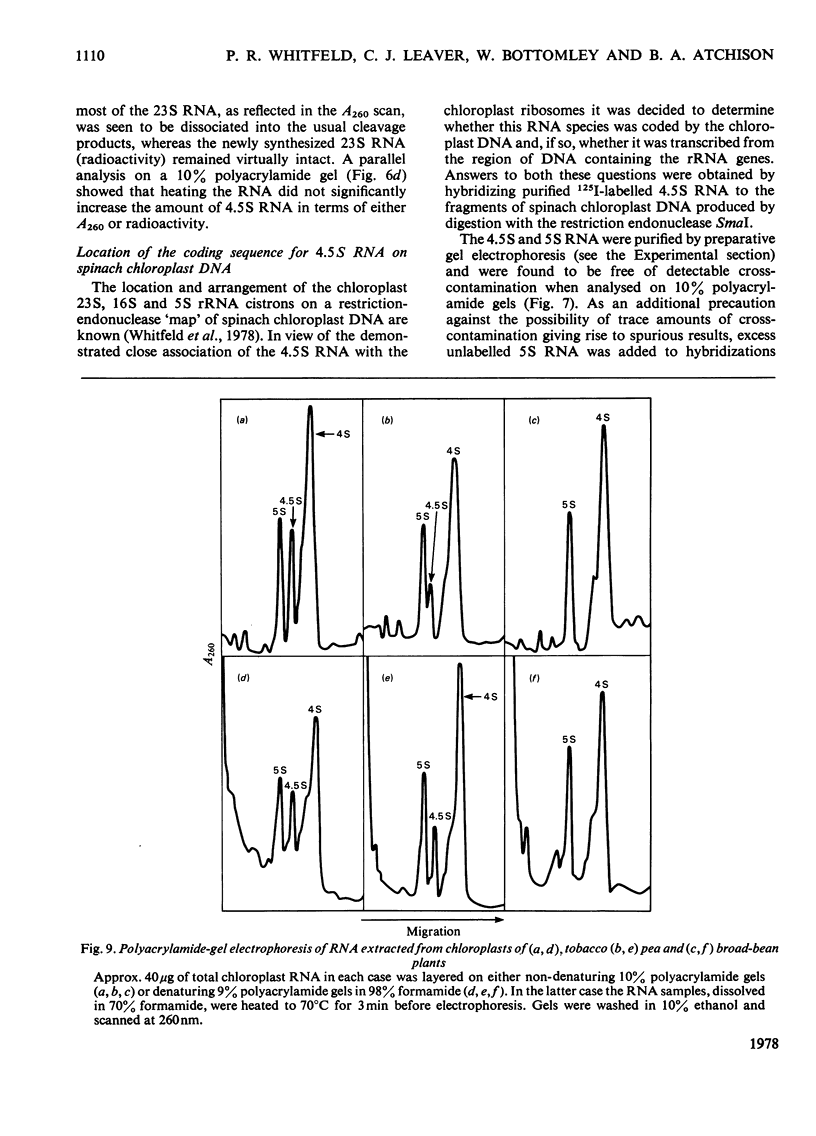
A species of RNA that migrates on 10% (w/v) polyacrylamide gels between 5S and 4S RNA was detected in spinach chloroplasts. This RNA (referred to as 4.5 S RNA) was present in amounts equimolar to the 5S RNA and its molecular weight was estimated to be approx. 33 000. Fractionation of the chloroplast components showed that the 4.5S RNA was associated with the 50 S ribosomal subunit and that it could be removed by washing the ribosomes with a buffer containing 0.01 M-EDTA and 0.5 M-KCl. It did not appear to be a cleavage product of the labile 23 S RNA of spinach chloroplast ribosomes. When 125I-labelled 4.5 S RNA was hybridized to fragments of spinach chloroplast DNA produced by SmaI restriction endonuclease, a single fragment (mol.wt. 1.15 times 10(6)) became labelled. The same DNA fragment also hybridized to chloroplast 5 S RNA and part of the 23 S RNA. It was concluded that the coding sequence for 4.5 S RNA was part of, or immediately adjacent to, the rRNA-gene region in chloroplast DNA . A comparable RNA species was observed in chloroplasts of tobacco and pea leaves.
Full text
PDF
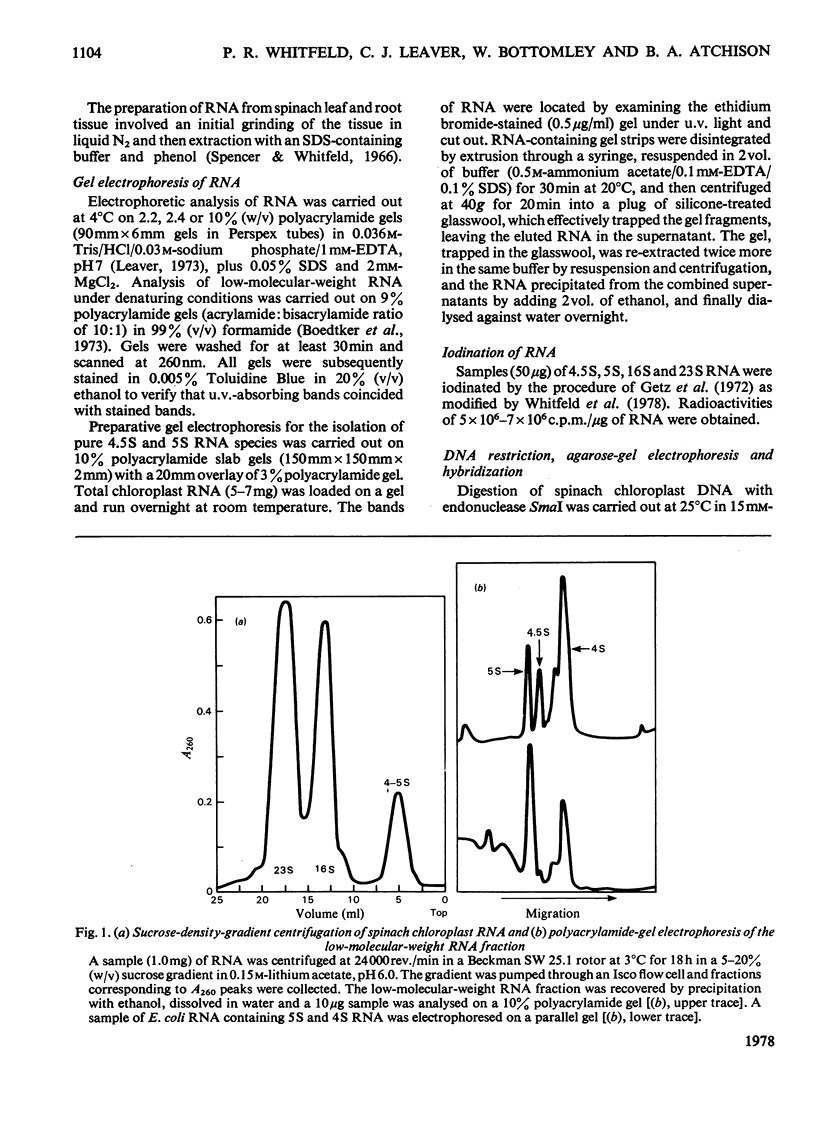
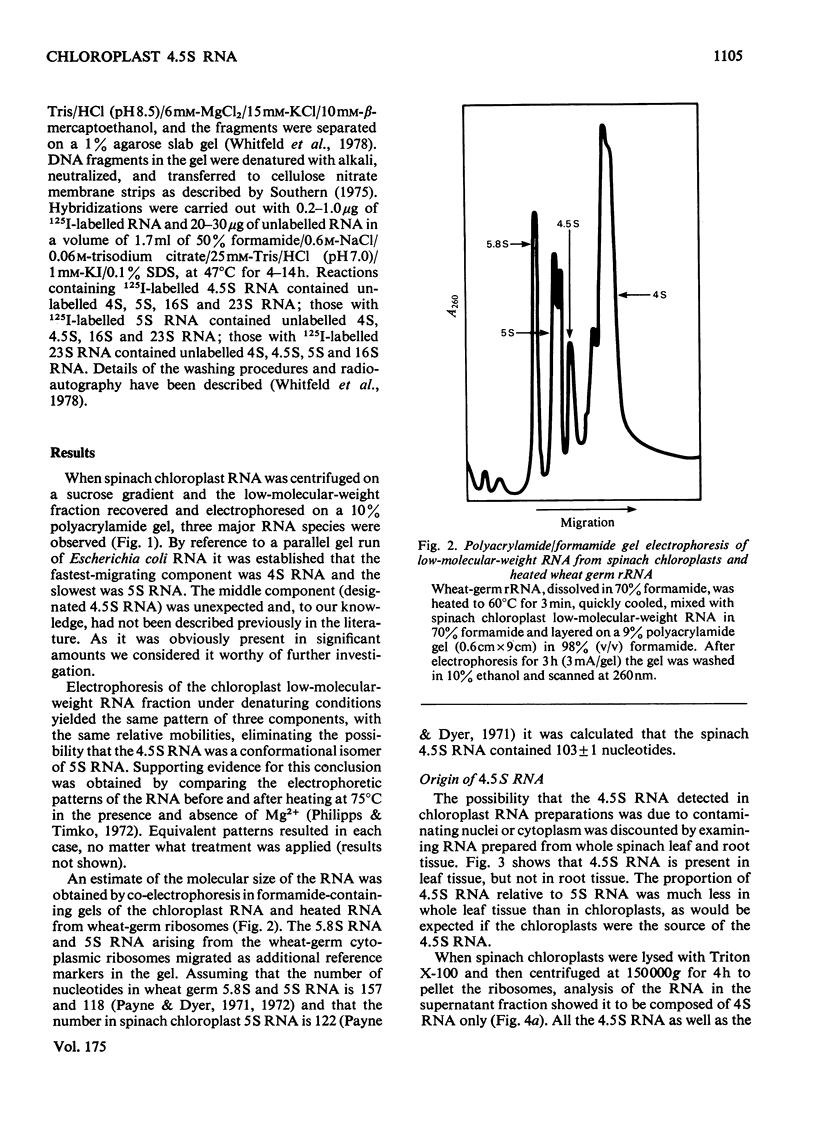

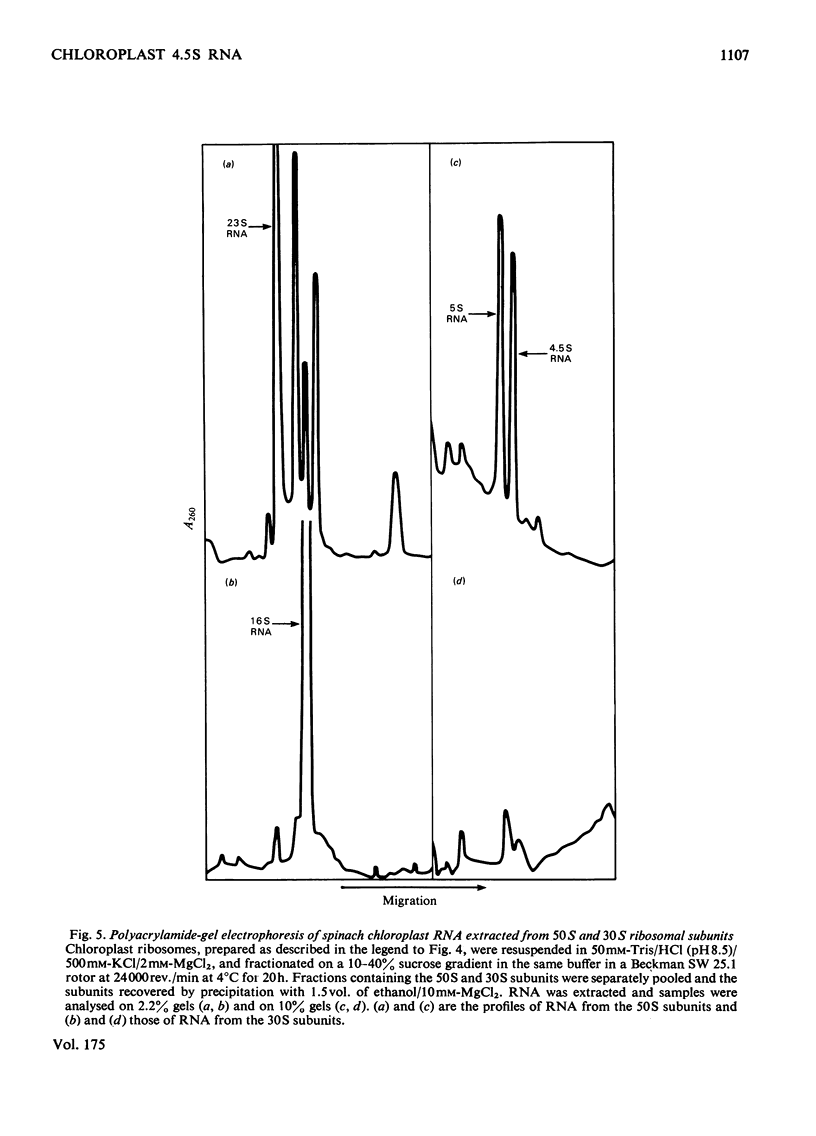
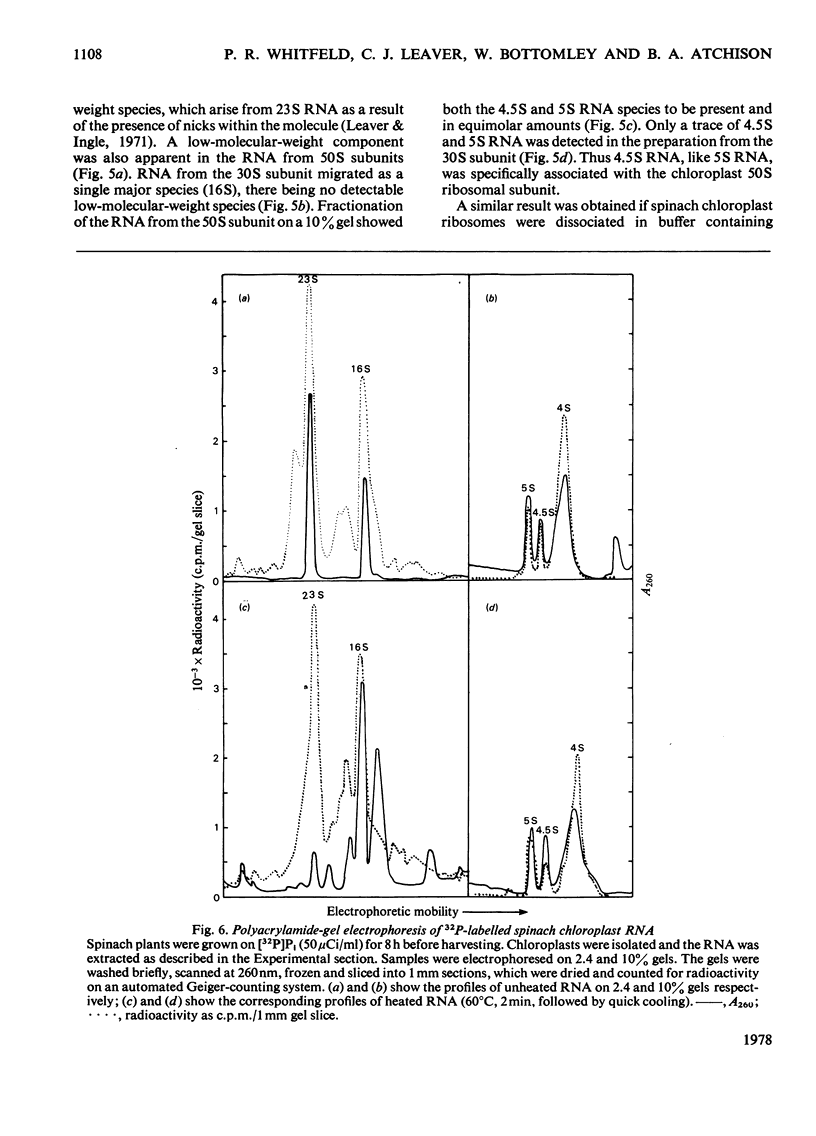


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boedtker H., Crkvenjakov R. B., Dewey K. F., Lanks K. Some properties of high molecular weight ribonucleic acid isolated from chick embryo polysomes. Biochemistry. 1973 Oct 23;12(22):4356–4360. doi: 10.1021/bi00746a009. [DOI] [PubMed] [Google Scholar]
- Bothwell A. L., Garber R. L., Altman S. Nucleotide sequence and in vitro processing of a precursor molecule to Escherichia coli 4.5 S RNA. J Biol Chem. 1976 Dec 10;251(23):7709–7716. [PubMed] [Google Scholar]
- Dyer T. A., Leech R. M. Chloroplast and cytoplasmic low-molecular-weight ribonucleic acid components of the leaf of Vicia faba L. Biochem J. 1968 Feb;106(3):689–698. doi: 10.1042/bj1060689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getz M. J., Altenburg L. C., Saunders G. F. The use of RNA labeled in vitro with iodine-125 in molecular hybridization experiments. Biochim Biophys Acta. 1972 Dec 22;287(3):485–494. doi: 10.1016/0005-2787(72)90293-6. [DOI] [PubMed] [Google Scholar]
- Griffin B. E. Studies and sequences of Escherichia coli 4.5 S RNA. J Biol Chem. 1975 Jul 25;250(14):5426–5437. [PubMed] [Google Scholar]
- Herrmann R. G., Bohnert H. J., Kowallik K. V., Schmitt J. M. Size, conformation and purity of chloroplast DNA of some higher plants. Biochim Biophys Acta. 1975 Jan 20;378(2):305–317. doi: 10.1016/0005-2787(75)90118-5. [DOI] [PubMed] [Google Scholar]
- Ingle J. Synthesis and Stability of Chloroplast Ribosomal-RNA's. Plant Physiol. 1968 Sep;43(9):1448–1454. doi: 10.1104/pp.43.9.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver C. J., Ingle J. The molecular integrity of chloroplast ribosomal ribonucleic acid. Biochem J. 1971 Jun;123(2):235–243. doi: 10.1042/bj1230235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver C. J. Molecular integrity of chloroplast ribosomal ribonucleic acid. Biochem J. 1973 Sep;135(1):237–240. doi: 10.1042/bj1350237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund E., Dahlberg J. E. Spacer transfer RNAs in ribosomal RNA transcripts of E. coli: processing of 30S ribosomal RNA in vitro. Cell. 1977 Jun;11(2):247–262. doi: 10.1016/0092-8674(77)90042-3. [DOI] [PubMed] [Google Scholar]
- Payne P. I., Dyer T. A. Characterization of cytoplasmic and chloroplast 5S ribosomal ribonucleic acid from broad-bean leaves. Biochem J. 1971 Aug;124(1):83–89. doi: 10.1042/bj1240083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne P. I., Dyer T. A. Phytochrome and temperature relations in Lactuca sativa L. Grand Rapids seed germination after thermo-dormancy. Nat New Biol. 1972 Feb 2;235(57):145–147. [PubMed] [Google Scholar]
- Philipps G. R., Timko J. L. Simple method for the characterization of 5S RNA. Anal Biochem. 1972 Jan;45(1):319–325. doi: 10.1016/0003-2697(72)90035-8. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spencer D., Whitfeld P. R. Ribonucleic acid synthesizing activity of spinach chloroplasts and nuclei. Arch Biochem Biophys. 1967 Aug;121(2):336–345. doi: 10.1016/0003-9861(67)90085-9. [DOI] [PubMed] [Google Scholar]
- Spencer D., Whitfeld P. R. The nature of the ribonucleic acid of isolated chloroplasts. Arch Biochem Biophys. 1966 Nov;117(2):337–346. doi: 10.1016/0003-9861(66)90421-8. [DOI] [PubMed] [Google Scholar]
- Whitfeld P. R., Higgins T. J. Occurrence of short particles in beans infected with the cowpea strain of TMV. I. Purification and characterization of short particles. Virology. 1976 Jun;71(2):471–485. doi: 10.1016/0042-6822(76)90375-5. [DOI] [PubMed] [Google Scholar]



