Abstract
Preclinical models of breast cancer that better predict patient-specific drug responses are critical for expanding the clinical utility of targeted therapies, including for inhibitors of poly(ADP-ribose) polymerase (PARP). Reprogramming primary cancer cells into human induced pluripotent stem cells (hiPSCs) recently emerged as a powerful tool to model drug response phenotypes, but its use to date has been limited to hematopoietic malignancies. We designed an optimized reprogramming methodology to generate breast cancer-derived hiPSCs (BC-hiPSCs) from nine patients representing all major subtypes of breast cancer. BC-hiPSCs retain patient-specific oncogenic variants, including variants unique to individual tumor subclones. Additionally, we developed a protocol to differentiate BC-hiPSCs into mammary epithelial cells and mammary-like organoids for in vitro disease modeling, including drug response phenotyping. Using these tools, we demonstrated that BC-hiPSCs can be used to screen for differential sensitivity to PARP inhibitors and mechanistically investigated the causal genetic variant driving drug sensitivity in one patient.
Subject terms: Breast cancer, Stem cells
Introduction
There is a growing consensus that the next generation of disease models for cancer should prioritize patient-derived models that better recapitulate the cellular and genetic complexity of human tumors. In line with these efforts, somatic cell reprogramming has recently emerged as a novel approach for modeling cancer1. Direct reprogramming of primary cancer cells into human induced pluripotent stem cells (hiPSCs) provides a means to capture the genome of individual cancer cells in a pluripotent state. hiPSCs have been generated from patients with a range of hematopoietic malignancies, including acute and chronic myeloid leukemias2–14. These hiPSCs retain somatic variants present in primary leukemia cells and display a variety of leukemic phenotypes when re-differentiated into hematopoietic cells, including gene-dependencies, differentiation blockades, and drug response phenotypes. Reprogramming cancer cells from solid tumor cells, including breast cancer, thus far represents an untapped opportunity for cancer disease modeling.
Breast cancer patients exhibit phenotypically distinct tumors that differ in their cellular, molecular, and genetic features, highlighting the need for disease models that capture this disease complexity. Intrinsic subtypes of breast cancer are primarily delineated through the tumor cell expression of three receptors, estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2), with a subset of triple negative breast tumors lacking expression of each receptor15,16. Expression of these receptors guides clinical treatment with endocrine or anti-HER2 therapies. In addition, a limited number of targeted therapies are now in clinical use for specific subsets of breast cancer patients, including the use of poly-ADP(ribose) polymerase (PARP) inhibitors for individuals who carry pathogenic variants in BRCA1 or BRCA217–19. However, clinical use of targeted therapies is limited by an insufficient understanding of drivers of drug resistance as well as a small fraction of breast cancer patients who currently qualify for these drugs20. While the landscape of somatic variants in breast cancer is well described, there is an ongoing need to mechanistically associate tumor genotypes with drug response phenotypes. Expanding the utility of targeted therapies in breast cancer thus relies on preclinical disease models that capture the genetic variability of human breast tumors and can be used to screen for patient-specific drug responses and identify biomarkers for drug sensitivity.
Cancer drug screening and target validation has traditionally relied on immortalized cancer cell lines, followed by subsequent validation and refinement in mouse models21. However, immortalized cancer cell lines suffer from several limitations: they are derived from late-stage tumor cells, undergo adaptation in culture, and can be composed of poorly defined, heterogenous cell populations, all of which limit their reliability for early-stage drug discovery22. Primary tumor cells provide a more physiologically relevant disease modeling platform, but their use is restricted by a limited lifespan in culture and incompatibility with genetic manipulation. Tumor-derived hiPSCs integrate the patient-specific features of primary cells with the experimental compatibility of immortalized cell lines and have been successfully used to probe drug response phenotypes in leukemia. In one instance, genetically distinct hiPSCs derived from subclonal tumor cells of an individual with acute myeloid leukemia identified a population of KRAS wild-type cells that displayed resistance to MEK inhibitors in vitro and correctly predicted clinical disease relapse in this patient8. In this report and others, differential drug sensitivity was dependent on the re-differentiation of hiPSCs into hematopoietic cells, supporting the conclusion that cell identity and differentiation status is critical for modeling drug response phenotypes8,12,14. Additionally, the compatibility of tumor-derived hiPSCs with genetic and chemical manipulation has been exploited to investigate the contributions of patient-specific variants to leukemic phenotypes, including response to existing and novel targeted therapies for leukemia4,6,10,11. We postulated that an hiPSC-based model of breast cancer could similarly be used to interrogate patient-specific responses to PARP inhibitors.
Here we apply tumor cell reprogramming to modeling breast cancer (Fig. 1A). We designed an optimized methodology for high-efficiency reprogramming of primary breast cancer cells and used it to generate hiPSC lines from nine breast cancer patients (BC-hiPSCs), including those with luminal hormone receptor-positive, HER2-enriched, and triple-negative breast tumors. We identified patient-specific somatic variants in BC-hiPSC lines, including genetically distinct lines derived from subclonal tumor cells of two of the breast cancer patients. Next, we demonstrated that hiPSCs can be differentiated into mammary epithelial cells and mammary-like organoids, which share gene and protein expression features with primary epithelial cells and provide a suitable system to model breast tumor phenotypes. Finally, we demonstrated that BC-hiPSCs derived from breast tumors with pathogenic BRCA1 or BRCA2 variants exhibit pronounced sensitivity to PARP inhibitors. Using CRISPR gene editing, we mechanistically investigated the contributions of the BRCA1 gene and the patient-specific BRCA1 c.68_69delAG genotype to the PARP inhibitor sensitivity phenotype. Overall, this work introduces BC-hiPSCs as a novel preclinical model for breast cancer that is well suited to investigate patient-specific responses to anti-cancer drugs, a fundamental goal in precision oncology.
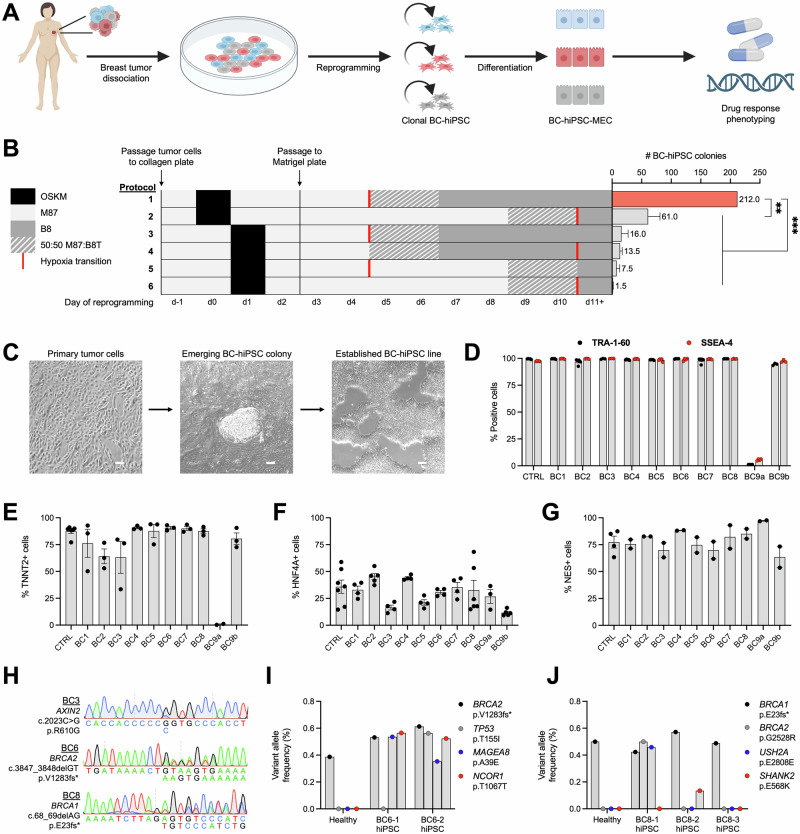
Fig. 1. Primary breast cancer cells can be reprogrammed into BC-hiPSCs that retain patient- and subclone-specific genetic variants.
A Overview for establishing an hiPSC model of breast cancer: primary breast tumor cells are dissociated and reprogrammed into hiPSCs (BC-hiPSCs), followed by differentiation into mammary epithelial cells (BC-hiPSC-MECs) for drug response phenotyping. B Number of BC-hiPSC colonies generated following different reprogramming methodologies, where each row is an independent experiment (OSKM = OCT4/SOX2/KLF4/MYC expression; M87 = breast tumor cell media; B8T/B8 = hiPSC media; n = 2). C Representative phase-contrast images of primary breast cancer cells during reprogramming showing emergence of BC-hiPSC colony. D Flow cytometry assessment of TRA-1-60 and SSEA4 expression in control and BC-hiPSC lines (n = 3–5). E Flow cytometry assessment of TNNT2 expression in control and BC-hiPSC lines differentiated into cardiomyocytes (n = 3–6). F Flow cytometry assessment of HNF4A expression in control and BC-hiPSC lines differentiated into hepatocytes (n = 3–7). G Flow cytometry assessment of NES expression in control and BC-hiPSC lines differentiated into neural progenitor cells (n = 2-4). H Sanger sequencing of BC-hiPSCs for patient-specific variants in AXIN2 (BC3), BRCA2 (BC6), and BRCA1 (BC8). Variant allele frequency for variants in BC-hiPSCs and isogenic healthy cells from BC6 (I) and BC8 (J) patients determined by whole genome sequencing. n = experimental replicates, ANOVA, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.005, ****P ≤ 0.0001, ns = not significant. Scale bars represent 100 µm.
Results
Primary breast cancer cells can be reprogrammed into BC-hiPSCs that retain patient- and subclone-specific genetic variants
We obtained tumor tissue from breast cancer patients and used an enzymatic dissociation protocol to isolate primary breast tumor cells (Supplementary Fig. 1A)23. These cells exhibited an epithelial cell morphology and expressed the epithelial intermediate filament protein KRT18 (Supplementary Fig. 1A, B). Our initial attempts to reprogram breast tumor cells into hiPSCs using a non-integrating Sendai virus to transiently overexpress the canonical reprogramming factors (OCT4, SOX2, KLF4, and MYC)24 had limited success: breast tumor cells exhibited low reprogramming efficiency (yielding 1–2 BC-hiPSC colonies) and many tumor samples were refractory to reprogramming altogether. We therefore examined whether the reprogramming methodology could be optimized specifically for breast tumor cells. We discovered that altering the timing for both transcription factor overexpression and for transitioning cells to conditions that favor hiPSC growth had a profound impact on reprogramming efficiency, resulting in an over 100-fold increase in the number of BC-hiPSC colonies generated from a single breast tumor (Fig. 1B; Supplementary Fig. 1C).
We used this optimized reprogramming methodology to generate over 200 BC-hiPSC lines from the tumor cells of nine breast cancer patients (Fig. 1C; Table 1). The breast cancer patients selected for this study encompass a range of molecular subtypes of disease, including estrogen and progesterone receptor-positive, HER2-enriched, and triple-negative breast cancer (Table 1; Supplementary Data 1). In one individual with contralateral breast cancer (BC9), the second lesion was later diagnosed as a benign fibroadenoma (BC9b); however, we elected to retain this sample to detect possible differences between BC-hiPSCs derived from isogenic benign and malignant tumors. BC-hiPSCs exhibited characteristic morphology and growth patterns, including extended proliferation in compact, well-defined colonies (Supplementary Fig. 1D). We did not observe differences in the morphology, growth rate, or differentiation capacity of BC-hiPSCs up to 100 passages. We selected one BC-hiPSC line from each breast tumor sample for further pluripotency validation. These BC-hiPSCs expressed canonical pluripotency markers, including TRA-1-60, SSEA4, OCT4, and SOX2 (Fig. 1D; Supplementary Fig. 2A). Additionally, BC-hiPSCs could differentiate into cell types representing all three germ layer lineages—cardiomyocytes (mesoderm), hepatocytes (endoderm), and neural progenitor cells (ectoderm) (Fig. 1E–G; Supplementary Fig. 2B–D). Interestingly, one breast tumor sample (BC9a) only produced a single, partially reprogrammed hiPSC line. While the BC9a partially reprogrammed line displayed extended proliferation capacity and expressed the pluripotency transcription factors OCT4 and SOX2, it lacked expression of the pluripotency cell surface markers TRA-1-60 and SSEA4, did not grow in clearly defined colonies, and exhibited reduced differentiation capacity.
Table 1.
Summary of breast cancer patient recruitment and BC-hiPSC generation
| Breast cancer patient ID | ER/PR/HER2 status | BRCA status | # BC-hiPSC lines |
|---|---|---|---|
| BC1 | ER/PR + | 2 | |
| BC2 | ER + | 122 | |
| BC3 | ER/PR + | 16 | |
| BC4 | ER/PR + | 10 | |
| BC5 | ER/PR + | 21 | |
| BC6 | ER/PR + | BRCA2 p.V1283fs* | 13 |
| BC7 | ER/PR/HER2 + | 10 | |
| BC8 | Triple-negative | BRCA1 p.E23fs* | 3 |
| BC9a | Triple-negative | 1 | |
| BC9b | N/A (fibroadenoma) | 15 |
BRCA status includes known variants in BRCA1 and BRCA2 identified through clinical gene sequencing panels. BC9a and BC9b tissues were obtained from the same patient with contralateral breast cancer. ER estrogen receptor, PR progesterone receptor, HER2 human epidermal growth factor receptor 2.
Clinical genetic testing for three of the breast cancer patients identified germline variants in breast cancer susceptibility genes: AXIN2 (BC3), BRCA2 (BC6), and BRCA1 (BC8). We confirmed that these variants were retained in BC-hiPSCs generated from each tumor (Fig. 1H). Next, we performed whole genome sequencing of BC-hiPSCs and, when available, patient-matched cells from histologically normal breast tissue, to comprehensively assess the genomic landscape of BC-hiPSCs (Supplementary Data 2). BC-hiPSCs harbor somatic variants in genes that are recurrently mutated in breast cancer, including TP53, MAGEA8, NCOR1, BRCA2, USH2A, SHANK2, MAP3K13, DNAH2, CHD1, and DNAH5 (Fig. 1I, J; Supplementary Fig. 1E, F)25. Notably, by sequencing multiple BC-hiPSC lines from the same tumor, we identified somatic variants unique to individual BC-hiPSC lines, including the TP53 p.T155I variant in BC6-2 hiPSCs and the BRCA2 p.G2528R variant in BC8-1 hiPSCs (Fig. 1I, J). This indicates that BC-hiPSCs, which are clonally derived from individual cancer cells, can capture genetically distinct subclones within heterogenous tumors. Overall, we demonstrate for the first time that primary breast cancer cells can be successfully reprogrammed into BC-hiPSCs which retain both patient- and subclone-specific genomic variants.
Establishment of an hiPSC-MEC differentiation protocol
Reprogramming results in global changes in gene expression that help initiate and sustain a pluripotency state26. Therefore, to model breast tumor phenotypes, we sought to re-differentiate hiPSCs into mammary epithelial cells (hiPSC-MECs) (Fig. 2A). The mammary gland is composed of two primary epithelial cell populations, luminal epithelial cells, the cell-of-origin for breast cancer, and contractile myoepithelial cells27,28. Differentiated hiPSC-MECs exhibited a cobblestone-like epithelial cell morphology and expressed several epithelial cell markers observed in the mammary epithelium, including KRT14, KRT18, and EPCAM (Fig. 2B–D). We assessed various cell culture conditions to optimize the overall viability and proliferative potential of hiPSC-MECs, including the basal media during differentiation and the surface coating and media for passaging differentiated cells (Supplementary Fig. 3A–C). Notably, culturing hiPSC-MECs in the presence of a rho-associated protein kinase inhibitor extended the proliferation of these cells to at least 3 passages (Supplementary Fig. 3D–F).
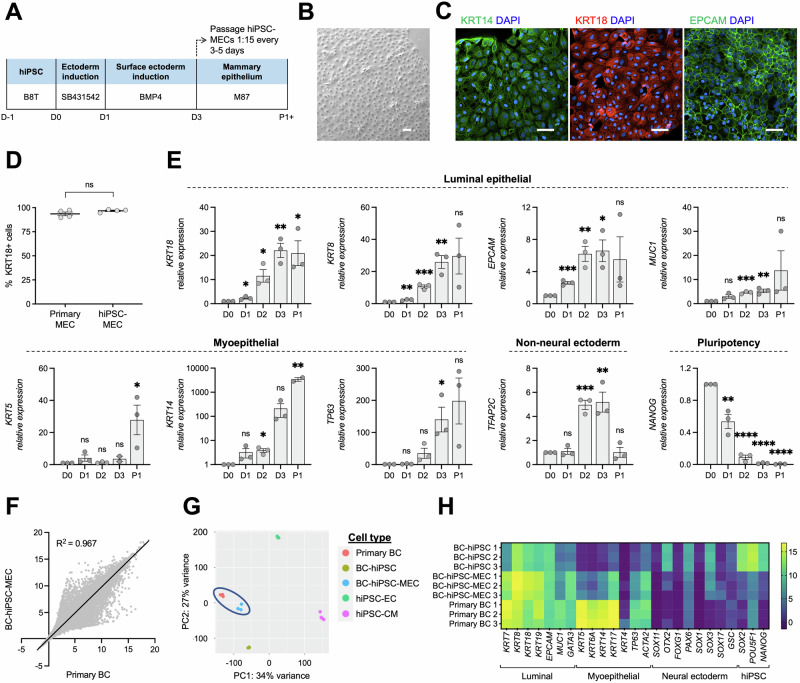
Fig. 2. Establishment of an hiPSC-MEC differentiation protocol.
A Schematic of hiPSC-MEC differentiation (SB431542 = DMEM/F12 with 10 µM SB431542 and 20 µg/mL insulin; BMP4 = DMEM/F12 with 10 ng/mL BMP4 and 20 µg/mL insulin; M87 = MEC media). B Phase contrast image of hiPSC-MECs. C Immunofluorescence staining for KRT14, KRT18, and EPCAM in hiPSC-MECs. D Flow cytometry assessment of KRT18 expression in primary MECs and hiPSC-MECs (n = 4). E Expression of select luminal epithelial, myoepithelial, non-neural ectoderm, and pluripotency genes at each day of MEC differentiation (D0-3) and after one passage (P1; cells collected 3 days post-passage) by RT-PCR, normalized to D0 (n = 3). F Correlation of RNA-seq gene expression data between primary breast cancer cells (primary BC) and BC-hiPSC-MECs. G PCA plot for primary BCs, BC-hiPSCs, and BC-hiPSC-MECs, as well as hiPSCs differentiated into endothelial cells (hiPSC-ECs) and cardiomyocytes (hiPSC-CMs). H Heatmap of RNA-seq data for expression of luminal epithelial, myoepithelial, neural ectoderm, and pluripotency (hiPSC) genes in BC-hiPSCs, BC-hiPSC-MECs, and primary BCs. n = experimental replicates, unpaired Student’s T-test, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.005, ****P ≤ 0.0001, ns = not significant. Scale bars represent 100 µm.
We next examined changes in mammary epithelial cell gene expression throughout differentiation. We observed upregulation of both luminal epithelial (KRT18, KRT8, EPCAM, MUC1) and myoepithelial (KRT5, KRT14, TP63) cell genes, which generally peaked at the final day of differentiation (day 3) and were maintained for at least one cell passage, with concordant downregulation of the pluripotency marker NANOG (Fig. 2E). Expression of TFAP2C, a transcription factor expressed during early surface ectoderm commitment29 was reduced at the first passage of hiPSC-MECs, suggesting cells undergo further lineage commitment at this time. Finally, we assessed global gene expression patterns in patient-derived BC-hiPSC-MECs using RNA-seq. BC-hiPSC-MECs are transcriptionally similar to the primary breast cancer cells from which they are derived, indicated by correlation in overall gene expression (R2 = 0.967), clustering in a principal component analysis, and comparable expression of select mammary epithelial cell-related genes (Fig. 2F–H). Importantly, primary breast cancer cells and BC-hiPSC-MECs clustered together and separately from undifferentiated BC-hiPSCs, suggesting that reprogramming breast cancer cells into BC-hiPSCs results in global gene expression changes that are reversed when BC-hiPSCs undergo differentiation into mammary epithelial cells (Fig. 2G). Comparing the expression of luminal and myoepithelial cell genes in BC-hiPSC-MECs, we observed higher expression of the luminal epithelial cell gene set, suggesting that BC-hiPSCs adopt a luminal epithelial-like cell identity during differentiation. We also confirmed that mammary epithelial cell differentiation does not increase expression of genes common to neural cells, the predominant ectodermal lineage (Fig. 2H). Altogether, these data present a reliable protocol to differentiate hiPSCs into luminal mammary epithelial cells and demonstrate that differentiation restores the gene expression profile that is altered during reprogramming.
Application of BC-hiPSCs in spheroid tumor models
We next sought to examine whether our mammary epithelial cell differentiation could be adapted for more complex, three-dimensional models of breast cancer. While simple monolayer cultures are advantageous for high throughput assays, including genetic and chemical screening, organoid models of breast cancer better recapitulate the physiological conditions of human tumors. We generated hiPSC-derived mammary organoids by first clustering hiPSCs into embryoid bodies before initiating mammary epithelial differentiation. We show that BC-hiPSCs from each breast tumor form mammary-like organoids in suspension culture or embedded in a Matrigel-based extracellular matrix (Supplementary Fig. 4A, B). We did not observe differences in the overall structure or growth of organoids derived from each BC-hiPSC line, with the exception of the BC9a partially reprogrammed line, which produced large, unstructured organoids. Additionally, while BC-hiPSC-derived mammary organoids retain KRT14 and EPCAM expression, these organoids consistently exhibited minimal KRT18 expression, possibly suggesting a loss of luminal cell identity in favor of a myoepithelial cell phenotype (Supplementary Fig. 4C).
Next, we assessed whether BC-hiPSC-MECs can be used in a mammosphere formation assay, another spheroid-based assay that assesses a cell’s propensity for anchorage-independent growth and tumor initiation. Adherent BC-hiPSC-MECs dissociated into single cells and transferred to low attachment culture conditions formed mammosphere structures after 7 days of growth (Supplementary Fig. 4D). We observed marginal differences in the mammosphere formation efficiency (MFE) and size of mammospheres formed by BC-hiPSC-MECs from each breast tumor, again with the exception of the BC9a partially reprogrammed line (Supplementary Fig. 4E, F). BC9a-hiPSC-MECs exhibited a higher MFE and larger mammosphere size relative to BC-hiPSC-MECs derived from all other tumors, as well as hiPSC-MECs derived from BC9 patient-matched healthy epithelial cells and BC9b tumor cells of the contralateral fibroadenoma (Supplementary Fig. 4E–H). There was not a consistent difference in MFE or mammosphere size observed between paired tumor- and healthy-derived hiPSC-MECs from three other breast cancer patients (Supplementary Fig. 4G, H). It is possible that a limited capacity for differentiation maintains the BC9a partially reprogrammed line in a relatively pluripotent state, which enables the more aggressive growth characteristics observed in organoid and mammosphere models.
BC-hiPSC-MECs with BRCA variants exhibit selective sensitivity to PARP inhibitors
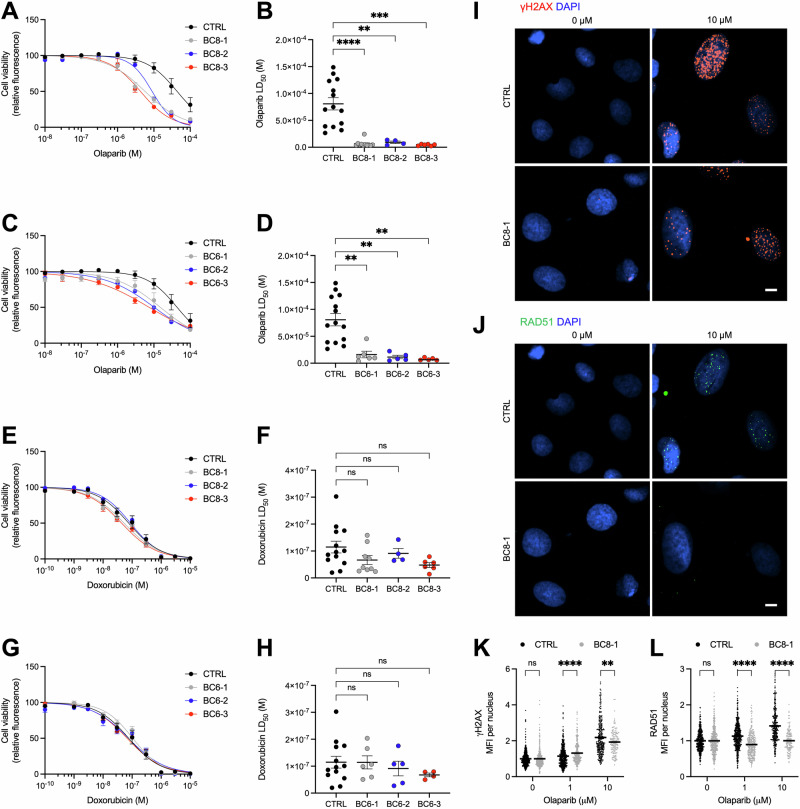
We next sought to determine whether BC-hiPSC-MECs provide a suitable platform to screen for, and ultimately predict, tumor-specific responses to anti-cancer drugs. We identified two breast cancer patients in our study with clinically actionable variants in BRCA1 (BC8) and BRCA2 (BC6) that can be targeted with PARP inhibitors (Fig. 1H). PARP inhibitors are synthetically lethal in BRCA-deficient genetic backgrounds due to the accumulation of DNA double-stranded breaks, which cannot be repaired using the high-fidelity, homology-directed repair pathway30,31. We found that BC8- and BC6-hiPSC-MECs were significantly more sensitive than control hiPSC-MECs to treatment with the PARP inhibitor olaparib in a cell viability assay, indicated by a 12.2- and 6.9-fold average reduction in the LD50, respectively (Fig. 3A–D). This drug response phenotype was confirmed using a second PARP inhibitor, talazoparib (Supplementary Fig. 5A–D). We did not observe a difference in olaparib or talazoparib drug sensitivity across three BC-hiPSC lines from each tumor, suggesting that subclonal BRCA2 p.G2528R (BC8-1) and TP53 p.T155I (BC6-2) variants do not alter the PARP inhibitor sensitivity phenotype in cells with additional BRCA variants. Additionally, the BC8- and BC6-hiPSC-MECs did not differ from control cells in their response to treatment with the anthracycline doxorubicin, confirming that the differential drug response is specific to PARP inhibitors (Fig. 3E–H). We next identified a BC-hiPSC line (BC4) without pathogenic variants in BRCA or other genes implemented in homology-directed repair (Supplementary Data 2)32. As expected, BC4-hiPSC-MECs were resistant to treatment with olaparib or talazoparib (Supplementary Fig. 6A–F).
Fig. 3. BC-hiPSC-MECs with BRCA variants exhibit selective sensitivity to PARP inhibitors.
Effect of olaparib treatment (6 days) on control (CTRL) hiPSC-MEC and BC8 hiPSC-MEC viability indicated by resazurin-based kill curve (A) and LD50 (B) (n = 4–14). Effect of olaparib treatment (6 days) on CTRL hiPSC-MEC and BC6 hiPSC-MEC viability indicated by resazurin-based kill curve (C) and LD50 (D) (n = 5–14). Effect of doxorubicin treatment (3 days) on CTRL hiPSC-MEC and BC8 hiPSC-MEC viability indicated by resazurin-based kill curve (E) and LD50 (F) (n = 4–13). Effect of doxorubicin treatment (3 days) on CTRL hiPSC-MEC and BC6 hiPSC-MEC viability indicated by resazurin-based kill curve (G) and LD50 (H) (n = 4–13). Immunofluorescence staining for γH2AX (I) and RAD51 (J) in CTRL hiPSC-MECs and BC8-1 hiPSC-MECs treated with indicated concentration of olaparib or DMSO (0 μM), representative images shown. Quantification of γH2AX (K) and RAD51 (L) staining intensity in CTRL hiPSC-MECs and BC8-1 hiPSC-MECs treated with the indicated concentration of olaparib or DMSO (0 μM), normalized to 0 μM (n ≥ 140 cells/condition). CTRL = 3 control cell lines. LD50 values (mean and SEM) are presented in Supplementary Table 1. n = experimental replicates, unpaired Student’s T-test, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.005, ****P ≤ 0.0001, ns = not significant. Scale bars represent 10 µm.
To further assess whether the difference in PARP inhibitor drug sensitivity is due to the lack of functional BRCA protein, we assessed the DNA damage repair capacity of BC8-hiPSC-MECs. Short-term olaparib treatment at near-LD50 concentrations resulted in the accumulation of γH2AX foci, indicative of DNA double-stranded breaks, in control hiPSC-MECs and BC8-hiPSC-MECs irrespective of BRCA1 variant status (Fig. 3I, K). However, formation of RAD51 foci was reduced in BC8-hiPSC-MECs relative to control cells, indicating a deficiency in the homology-directed repair pathway (Fig. 3J, L). BC8-hiPSC-MECs were similarly unable to induce RAD51 expression following treatment with talazoparib (Supplementary Fig. 5E–H). These data confirm existing reports that cells with BRCA1 variants are unable to initiate high-fidelity repair of DNA double-stranded breaks that are produced by PARP inhibitors, contributing to the selective toxicity of these drugs.
Loss of BRCA1 sensitizes hiPSC-MECs to PARP inhibitors
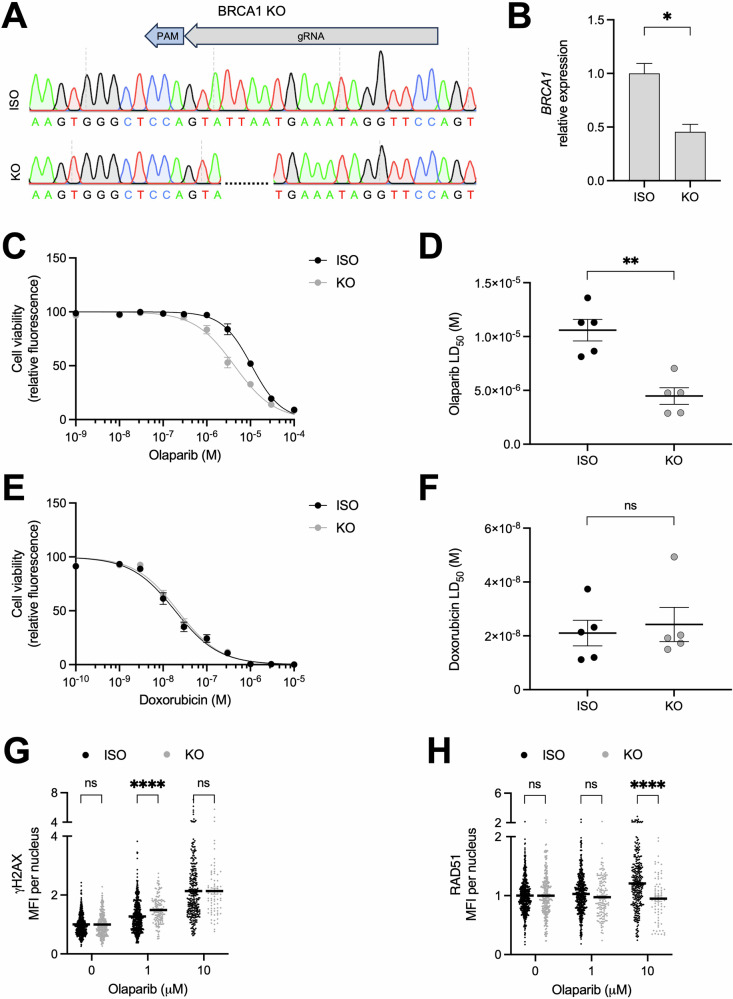
While the relationship between BRCA deficiency and response to PARP inhibitors is well established, the clinical utility of these drugs is limited by high rates of therapy resistance and an incomplete understanding of the role of non-BRCA genes in the response to PARP inhibitors. As such, there is a prevailing need to better understand the genetic determinants that predict clinical response to these drugs. hiPSCs provide a platform to directly evaluate the contributions of individual genes and genetic variants to a drug response phenotype. To first assess whether loss of BRCA1 is sufficient to sensitize hiPSC-MECs to PARP inhibitors, we generated a BRCA1 knockout hiPSC line using CRISPR/Cas9 gene editing (Fig. 4A, B). Loss of BRCA1 increased the sensitivity of hiPSC-MECs to olaparib treatment without altering the response to doxorubicin (Fig. 4C–F). Additionally, while loss of BRCA1 had a marginal effect on γH2AX expression in olaparib-treated hiPSC-MECs, RAD51 expression was significantly reduced in these cells, confirming that BRCA1 is necessary for homology-directed repair (Fig. 4G, H). The role of BRCA1 in altering the PARP inhibitor drug response was replicated by treatment with talazoparib (Supplementary Fig. 7A–D).
Fig. 4. Loss of BRCA1 sensitizes hiPSC-MECs to PARP inhibitors.
A Sanger sequencing of unedited isogenic (ISO) and BRCA1 knockout (KO) hiPSC lines. gRNA target and PAM site indicated. B BRCA1 expression in ISO and BRCA1 KO hiPSC lines by RT-PCR, normalized to ISO (n = 3). Effect of olaparib treatment (6 days) on ISO and BRCA1 KO hiPSC-MEC viability indicated by resazurin-based kill curve (C) and LD50 (D) (n = 5). Effect of doxorubicin treatment (3 days) on ISO and BRCA1 KO hiPSC-MEC viability indicated by resazurin-based kill curve (E) and LD50 (F) (n = 5). Quantification of γH2AX (G) and RAD51 (H) staining intensity in ISO and BRCA1 KO hiPSC-MECs treated with indicated concentration of olaparib or DMSO (0 μM), normalized to 0 μM (n ≥ 74 cells/condition). LD50 values (mean and SEM) are presented in Supplementary Table 1. n = experimental replicates, unpaired Student’s T-test, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.005, ****P ≤ 0.0001, ns not significant.
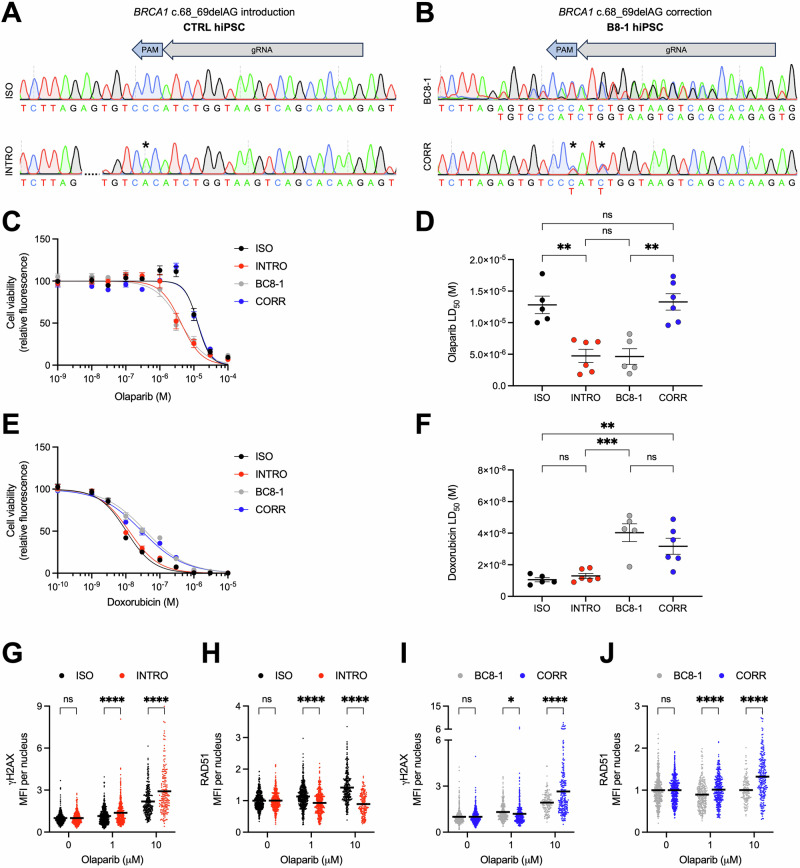
The patient-specific BRCA1 c.68_69delAG variant is necessary and sufficient for PARP inhibitor sensitivity in BC8-hiPSC-MECs
To further investigate whether the BC8 patient-specific BRCA1 c.68_69delAG variant alone drives the PARP inhibitor sensitivity phenotype, we introduced and corrected this variant in control hiPSC and BC8-hiPSC lines, respectively (Fig. 5A, B). Introduction of the BRCA1 c.68_69delAG variant was sufficient to sensitize control hiPSC-MECs to treatment with olaparib, without altering the response to doxorubicin (Fig. 5C–F). Additionally, introducing the BRCA1 variant prevented control cells from utilizing the RAD51-dependent homology-directed repair pathway (Fig. 5G, H). Conversely, correcting the BRCA1 c.68_69delAG variant in BC8-hiPSCs decreased olaparib sensitivity to levels observed in control cells (Fig. 5C–F) and restored the cells’ ability to repair DNA using homology-directed repair (Fig. 5I, J). The necessity and sufficiency of this BRCA1 variant for PARP inhibitor sensitivity was confirmed by treatment with talazoparib (Supplementary Fig. 7E–J). These data support the clinical utility of PARP inhibitors for the BC8 patient and other breast cancer patients with the BRCA1 c.68_69delAG variant, including the 1% of individuals of Ashkenazi Jewish ancestry who carry this founder variant33.
Fig. 5. The patient-specific BRCA1 c.68_69delAG variant is necessary and sufficient for PARP inhibitor sensitivity in BC8-hiPSC-MECs.
A Sanger sequencing of unedited isogenic control (ISO) hiPSC line and hiPSC line with BRCA1 c.68_69delAG variant introduced (INTRO). gRNA target, PAM site, and silent mutation (*) indicated. B Sanger sequencing of unedited BC8-1 hiPSC line and BC8-1 hiPSC line with BRCA1 c.68_69delAG variant corrected (CORR). gRNA target, PAM site, and silent mutations (*) indicated. Effect of olaparib treatment (6 days) on ISO, INTRO, BC8-1, and CORR hiPSC-MEC viability indicated by resazurin-based kill curve (C) and LD50 (D) (n = 5–6). Effect of doxorubicin treatment (3 days) on ISO, INTRO, BC8-1, and CORR hiPSC-MEC viability indicated by resazurin-based kill curve (E) and LD50 (F) (n = 5-6). Quantification of γH2AX (G) and RAD51 (H) staining intensity in ISO and INTRO hiPSC-MECs treated with indicated concentration of olaparib or DMSO (0 μM), normalized to 0 μM (n ≥ 174 cells/condition). Quantification of γH2AX (I) and RAD51 (J) staining intensity in BC8-1 and CORR hiPSC-MECs treated with indicated concentration of olaparib or DMSO (0 μM), normalized to 0 μM (n ≥ 140 cells/condition). LD50 values (mean and SEM) are presented in Supplementary Table 1. n = experimental replicates, unpaired Student’s T-test, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.005, ****P ≤ 0.0001, ns not significant.
Discussion
This work lays the foundation for BC-hiPSCs as a novel disease modeling platform for breast cancer. By reprogramming primary breast cancer cells, we’ve demonstrated for the first time that bona fide hiPSCs can be generated from primary cancer cells of any solid tumor. While hiPSC-like cells were previously generated from pancreatic adenocarcinoma cells, these cells were dependent on constitutive transcription factor overexpression34. The reprogrammed breast tumor cells in this study exhibit all fundamental features of hiPSCs: indefinite self-renewal in a pluripotent state, expression of canonical pluripotency markers, and the ability to differentiate into each of the three germ layer lineages.
Reprogramming fundamentally immortalizes individual cancer cells in a pluripotent state, providing a valuable resource for breast cancer research. While traditional immortalized breast cancer cell lines are readily available, these lines often exhibit genetic instability and undergo clonal selection resulting in heterogenous subpopulations of cells that confound efforts to associate cancer phenotypes with specific cell identities or genotypes22. Additionally, immortalized breast cancer cell lines are not available for all forms of breast cancer. Reprogramming breast cancer cells into hiPSCs provides an opportunity to expand the repertoire of tools available to study less common breast cancers that are not well represented by current disease models. For example, to our knowledge no existing immortalized cell lines are derived from males with breast cancer, as demonstrated with hiPSCs here (BC7). We also demonstrated that isogenic BC-hiPSC lines can be generated from benign and malignant tumors in the same individual (BC9), which could provide a unique tool to investigate genetic events underlying malignant transformation. Future efforts are critical to assess whether BC-hiPSCs can fulfill needs for disease models of other rare and underrepresented breast cancers, including inflammatory breast cancer and breast cancer in individuals of non-European ancestry.
Previous reports on the generation of hiPSCs from cancer cells of individuals with hematopoietic malignancies suggest that certain cell states and genomic alterations hinder or altogether preclude reprogramming6,10,11,34. This conclusion is largely based on anecdotal reports that certain cancer cells yield few or no hiPSC colonies, including acute myeloid leukemia cells with NPM1 mutations10. Using our optimized reprogramming protocol we did not observe notable differences in reprogramming efficiencies between patient-matched breast tumor and healthy cells, with the exception of the BC8 and BC9a tumors, which gave rise to few hiPSC colonies or only partially reprogrammed lines, respectively, despite multiple reprogramming attempts. The low reprogramming efficiency for these cancer cells could be explained by specific oncogenic lesions in these tumors, the triple-negative breast cancer subtype, or due to prior exposure to neoadjuvant chemotherapy. Patient-matched cells from the contralateral fibroadenoma lesion in the BC9 patient (BC9b) did successfully reprogram into hiPSCs, suggesting a cancer-specific barrier to reprogramming in the paired, invasive carcinoma sample (BC9a).
While we were able to generate BC-hiPSCs from tumors encompassing multiple subtypes of breast cancer, including luminal, HER2-enriched, and triple-negative tumors, it remains to be seen whether certain genomic alterations or cell types alter a breast tumor’s reprogramming potential. Specific genetic alterations, including those observed in breast cancer, have a demonstrated impact on cellular reprogramming. Notably, loss of TP53 increases reprogramming efficiency, while deficiencies in certain Fanconi anemia genes hinder reprogramming35–39. Despite playing a role in Fanconi anemia, our work demonstrates that a pathogenic variant in BRCA2 (FANCD1) does not preclude reprogramming. Comprehensive genomic characterization, encompassing genetic variants, copy number changes, and chromosomal abnormalities, of starting primary breast tumor cells and their hiPSC progeny across a large sample of breast tumors will be necessary to better understand the extent to which genetic alterations in breast cancer impact reprogramming. Tailoring reprogramming methodologies to specific cancer types and oncogenic lesions, as demonstrated here and in previous reports for acute myeloid leukemia10, may be sufficient to overcome reprogramming barriers in otherwise difficult-to-reprogram breast cancer cells.
Genomic characterization of reprogrammed cells in this study indicates that hiPSCs can be generated from tumor cells with germline and somatic variants in common breast cancer predisposing genes (BRCA1, BRCA2) and somatically mutated genes (TP53). Sequencing longitudinal BC-hiPSC samples across multiple passages can provide additional insight into the genomic stability of these cell lines relative to the primary tumor cells from which they are derived. Breast cancer is a genomically complex disease, with somatic variants and copy number alterations reported in hundreds of genes16,25. With the exception of PIK3CA and TP53, genes with coding mutations occur at a low frequency (<15%) across all breast tumors (TCGA), complicating efforts to assign a “one size fits all” model for breast cancer and arguing for patient-specific disease models. Additionally, human breast tumors exhibit genetic and molecular intratumor heterogeneity, which can strongly influence disease phenotypes. The number of genetically distinct subclones varies considerably in different breast tumors (estimates ranging from 1 to over 20) and can change over time under selective pressures such as drug treatment40,41. We show that BC-hiPSC lines with distinct genetic variants can be generated from the same breast tumor, suggesting these lines were derived from unique tumor subclones. For example, 2 hiPSC lines from the BC6 tumor share all somatic variants except for a TP53 variant, suggesting the BC6-1 and BC6-2 hiPSC lines were generated from TP53 wild type and variant tumor subclones, respectively. BC-hiPSCs thus provide an unprecedented opportunity to directly study both patient- and subclone-specific disease phenotypes in breast cancer, including response to anti-cancer drugs.
We postulated that differentiating hiPSCs into mammary epithelial cells would provide a more relevant cell state for drug response phenotyping. Breast tumors arise from luminal epithelial cells at distinct stages of differentiation within the mammary epithelium27,28. However, primary breast epithelial cells have the propensity to adopt a myoepithelial cell phenotype when cultured in vitro, either through a phenotypic switch or through selection of pre-existing myoepithelial cells42,43. Consistent with these reports, we detected high expression levels of myoepithelial cell genes in cultured primary breast cancer cells by RNA-seq. In contrast, our gene expression data suggests hiPSC-MECs adopt a luminal cell identity, more closely resembling the human breast epithelial cells that undergo malignant transformation in breast cancer and providing a physiologically relevant system for modeling breast tumor phenotypes in vitro.
PARP inhibitors are an appealing class of drugs to study with an hiPSC disease model because they are one of few targeted therapies for breast cancer whose use is dictated by a patient’s genome. The PARP inhibitors olaparib and talazoparib are now part of the standard of care for breast cancer patients with germline BRCA1 or BRCA2 variants with locally advanced, metastatic, or high-risk/early-stage disease17–19. While BRCA variants are a genetic indicator for PARP inhibitor use, not all patients exhibit a sustained clinical response to these drugs20. Multiple genetic mechanisms of innate and acquired resistance to PARP inhibitors have been proposed, including selection for reversion mutations that restore the BRCA open reading frame and expression of hypomorphic alleles, highlighting the need to more thoroughly assess patient-specific genetic determinants of response to these drugs44–46. We show that BC-hiPSC-MECs derived from two patients with BRCA1 (BC8) or BRCA2 (BC6) variants are sensitive to treatment with PARP inhibitors at clinically relevant doses (Cmax olaparib = 17.5 μM; Cmax talazoparib = 43 nM). Comparing PARP inhibitor sensitivity in BC-hiPSC-MECs to drug responses in patient-matched, primary tumor-derived organoids and clinical outcomes following PARP inhibitor treatment is a critical next step in assessing the predictive power of this model. Limited PARP inhibitor drug response data available for breast and ovarian cancer patients who carry the specific BRCA1 or BRCA2 variants in this study indicate variable responses, ranging from complete response to refractory, progressive disease47–50. This clinical data is confounded by differences in prior treatment received by each patient, due to overlapping mechanisms of resistance for some drugs, including PARP inhibitors and platinum-based chemotherapy46. Of interest for future studies is whether BC-hiPSCs can detect these patient-specific differences in clinical response to PARP inhibitors.
A unique advantage of using BC-hiPSCs as a drug testing platform is the ability to examine the drug response of distinct disease subclones. Intratumor genetic heterogeneity is a well-established driver of therapy resistance in breast cancer, including to PARP inhibitors. Advances in single-cell DNA sequencing technologies offer new insight into the clonal architecture of breast tumors and longitudinal sequencing of tumors from pre- and post-treatment biopsies provides insight into treatment-induced clonal evolution41,51. Disease modeling with BC-hiPSCs builds on these techniques by providing an unprecedented opportunity to directly study drug response phenotypes of disease subclones without confounding genetic and cellular heterogeneity. For example, we show that acquisition of a secondary BRCA2 variant in the BRCA1 deficient, BC8-1 cell line does not alter the PARP inhibitor response phenotype, suggesting there is not an additive effect for PARP inhibitor sensitivity in cells that exhibit deficiencies in multiple homology-directed repair genes.
In addition to screening for patient- and subclone-specific drug responses, tumor-derived hiPSCs are well suited to mechanistically investigate genetic determinants of a drug response phenotype within the context of a tumor cell’s specific genetic background. We provide proof of concept that the BRCA1 c.68_69delAG variant is both necessary and sufficient for PARP inhibitor sensitivity in the BC8 patient tumor. This data supports the use of BC-hiPSCs to probe for additional genetic biomarkers of sensitivity to PARP inhibitors, including variants outside of BRCA genes. We identified variants associated with homologous recombination deficiency in three BC-hiPSC lines—FANCA (BC1), ATR (BC3), and FANCD2 (BC9) (Supplementary Data 2)32. The cell bank generated here thus provides a resource to examine synthetic lethality between PARP and non-BRCA genes, an important goal for expanding the clinical utility of PARP inhibitors. BC-hiPSCs can similarly be used to identify genetic determinants of response to other existing targeted therapies for breast cancer, including PI3K and AKT inhibitors, as well as novel targeted therapies identified through drug and genetic screens. Identifying genetic biomarkers that predict drug response outcomes is critical for tailoring clinical disease management to individuals with breast cancer.
While the overall model presented here is well suited for pharmacogenomic assays, there are a number of opportunities to build on this model. For example, the mammary epithelial cell hierarchy contains stem cells, progenitor cells, and mature luminal and myoepithelial cells28. Protocols that enable the isolation and characterization of mammary cells at each stage of differentiation will be critical to using BC-hiPSCs to study ongoing questions related to the cell-of-origin for breast cancer and whether certain tumor- or variant-specific phenotypes are restricted to defined mammary cell types. Additionally, our mammary epithelial cells do not express estrogen or progesterone receptors. Inducing hormone receptor expression during differentiation into luminal epithelial cells will provide a better resource to study hormone-dependent phenotypes, including response to endocrine therapies. Similarly, assessing whether BC-hiPSCs derived from HER2-positive tumors retain HER2 overexpression will be critical for modeling responses to trastuzumab and other anti-HER2 therapies. Furthermore, while we show that BC-hiPSCs can be differentiated into mammary-like organoids, these organoids do not fully capture the breast tumor microenvironment. This can be addressed by building more physiologically relevant organoids that incorporate immune and stromal cells and assessing the extent to which organoids derived from BC-hiPSCs mimic organoids derived from primary breast cancer tissue. In addition, directly comparing drug sensitivity phenotypes in BC-hiPSC organoids to primary tumor organoids is essential. Finally, examining the tumorigenicity of BC-hiPSCs and BC-hiPSC-MECs following implantation into immunodeficient mice is a critical first step in assessing the compatibility of hiPSCs with in vivo models of breast cancer. Altogether, the work here lays the foundation for a patient-specific, hiPSC-based model of breast cancer that can be used to investigate fundamental questions in breast cancer biology and precision oncology.
Methods
Human samples
De-identified breast tumor tissue was obtained from nine breast cancer patients (BC1-9) by the Pathology Core Facility of the Robert H. Lurie Comprehensive Cancer Center (RHLCCC) under protocols approved by the Northwestern University Institutional Review Board and in accordance with recognized ethical guidelines (Declaration of Helsinki). Written informed consent was obtained from all patients before surgery. When available, we also obtained histologically normal breast tissue from the sample margin furthest from the tumor. Additional information on breast cancer patients and tumor samples (patient characteristics, pathology characteristics, treatment received) is available in Table 1 and Supplementary Data 1.
Isolation and culture of human breast cancer cells
Breast tumor and histologically normal tissue was processed following established protocols23. Tissue was placed in DMEM (Corning, 10-013-CV) and immediately transferred to the lab for subsequent dissociation into single cells as follows (Supplementary Fig. 1A): visibly necrotic tissue and fat was removed, and the remaining breast tissue was finely minced using scalpels. Minced tissue was collected in digestion media containing 2 mg/mL collagenase type 3 (Worthington, LS004182) and 100 U/mL hyaluronidase (Sigma, H3884) in DMEM/F12 (Corning, 10-092-CM) supplemented with 20 mg/mL bovine serum albumin (BSA, GenDEPOT, A0100), 5 µg/mL recombinant human insulin (Gibco, A11382ij), 0.5 µg/mL hydrocortisone (Sigma, H2270), and 100 U/mL penicillin–streptomycin (Gibco, 15140122). Tissue was processed in digestion media overnight (12–18 h) at 37 °C on an orbital shaker (200 rpm). Overnight digestion produced epithelial cell clusters of ~100–200 µm diameter, which were separated from additional cellular components by differential centrifugation as follows: the overnight digestion solution was first centrifuged at 100 × g for 30 s and the supernatant was subsequently collected and centrifuged at 200 × g for 3 min. The pellets from each centrifugation were resuspended in TrypLE (Gibco, 12604-013) and gently pipetted for 1–5 min to further process cell clusters into single cells. The resultant cells were resuspended in dissociation wash buffer, HBSS (Corning, 21-023-CV) containing 10 mM HEPES (Corning, 25-060-CI) and 2% Cosmic Calf Serum (CCS, Cytiva, SH3008703HI), and centrifuged at 350 × g for 5 min. Cell pellets were resuspended in HBSS containing 5 U/mL Dispase II (Sigma, D4693) and 90 µg/mL DNase I (Sigma, 11284932001) and gently pipetted for 1 min. Dissociation wash buffer was added to the cell suspension and centrifuged at 350 × g for 5 min. The pelleted cells were then transferred to plates coated with 5 µg/cm2 collagen I (Corning, 354236) diluted in 20 mM acetic acid (Fisher Scientific, BP1185-500) and plated in M87 media23: DMEMF12 with 2% CCS, 10 µg/mL recombinant human insulin, 5.5 µg/mL recombinant human transferrin (In Vitria, 777TRF029), 6.7 ng/mL sodium selenite (Sigma, S5261), 1× GlutaMAX (Gibco, 35050061), 5 ng/mL EGF (Peprotech, AF-100-15), 0.3 µg/mL hydrocortisone, 0.5 ng/mL cholera toxin (Sigma, C8052), 5 nM 3,3′,5-triiodo-l-thyronine (Sigma, T6397), 0.5 nM β-Estradiol (Sigma, E2758), 5 µM isoproterenol (Sigma, I6504), 50 nM ethanolamine (Sigma, E9508), 50 nM O-phosphorylethanolamine (Sigma, P0503), and 100 U/mL penicillin–streptomycin. Media change with M87 media was performed 2–3 times per week. Confluent cells were passaged with TrypLE for 5 min at 37 °C, centrifuged at 300 × g for 5 min, and replated in M87 media.
Generation of hiPSC lines from breast cancer cells
Early passage (p < 3) primary breast tumor or healthy epithelial cells were used to generate hiPSC lines. 1 × 105 primary breast epithelial cells were plated in 1 well of a collagen I-coated 6-well plate in M87 media. The following day (day 0), reprogramming was initiated using the CytoTune 2.0 Sendai Reprogramming Kit (Invitrogen, A16518). Cells were transduced by adding 1.5 × 105 CIU of hKOS virus, 1.5 × 105 CIU of hc-MYC virus, and 9 × 104 CIU of hKLF4 virus in M87 media. 24 h post-infection (day 1), Sendai virus was removed by washing cells once before adding fresh M87 media. On day 3, cells were dissociated with TrypLE for 5 min at 37 °C, then passaged 1:2 in M87 media onto 2, 10-cm plates coated with Matrigel (Corning, 356234). On day 5, media was changed to a 50:50 mixture of M87 and the hiPSC growth media B8T (see “hiPSC culture”) and plates were transferred to Heracell VIOS 160i humidified incubators (Thermo) with 5% CO2 and 5% O2. Starting on day 7, media was changed daily to the hiPSC growth media B8. Visible hiPSC colonies formed around day 10. Between days 15–30, individual hiPSC colonies were manually picked and transferred to Matrigel-coated 48-well plates in B8T media for expansion.
Control hiPSC lines
Control hiPSC lines used in this study have been previously published and include GW143c2 (F), GW132c2B (F), and 19c3 (M)52,53. Control lines were derived from isolated peripheral blood mononuclear cells of healthy volunteers under protocols approved by the Northwestern University Institutional Review Board and with written informed consent obtained from all volunteers.
hiPSC culture
hiPSCs were maintained in a low-cost variant of B8 medium made in-house53–55 with daily media changes on 1:800 growth factor reduced Matrigel (Corning, 356234) diluted in DMEM. B8 was supplemented with 2 μM thiazovivin (MedChemExpress, HY13257), referred to as B8T, for the first 24 h after passage. hiPSC lines were passaged at a ratio of 1:15 every 4 days (achieving ~75% confluency) by dissociating cells in 0.5 mM EDTA (Corning, 46-034-Cl) diluted in DPBS (Corning, 20-031-CV) for 6 min at RT and replating cells in B8T. Cell lines were used for differentiation and subsequent experiments between passage 20 and 100. All established pluripotent lines and lines undergoing reprogramming were maintained at 37 °C in Heracell VIOS 160i humidified incubators (Thermo) with 5% CO2 and 5% O2. hiPSC lines undergoing differentiation were transferred to atmospheric O2 incubators. All cultures were routinely tested for mycoplasma using a MycoAlert PLUS Kit (Lonza, LT07-710) and a Varioskan LUX (Thermo) plate reader.
Mammary epithelial differentiation
hiPSC lines were differentiated into mammary epithelial cells according to our modified version of protocols previously described for surface ectoderm differentiation53,56–58. Briefly, hiPSCs were split at a 1:20 ratio using 0.5 mM EDTA as above and grown in B8T medium for 1 day reaching ~15% confluency. On the first day of differentiation (day 0), medium was replaced with DMEM/F12 supplemented with 20 µg/mL of recombinant human insulin and 10 μM of the transforming growth factor-β type I receptor inhibitor SB431542 (Cayman Chemical, 13031). On day 1, the medium was changed to DMEM/F12 supplemented with 20 µg/mL of recombinant human insulin and 10 ng/mL of BMP4 (Peprotech, 120-05ET). On day 3, epithelial cells were dissociated using TrypLE for 5 min at 37 °C, centrifuged at 300 × g for 5 min, and replated for subsequent assays in M87 media supplemented with 1 μM thiazovivin (M87 + T) on plates coated with 5 µg/cm2 collagen I diluted in 20 mM acetic acid.
Cardiac differentiation
hiPSC lines were differentiated into cardiomyocytes using a previously described RBAI protocol52,59. Briefly, hiPSC lines were grown in B8 medium for 4 days until reaching ~75% confluency. On the first day of differentiation (day 0), B8 medium was changed to R6C: RPMI 1640 (Corning, 10-040-CM) supplemented with 6 μM of glycogen synthase kinase 3-b inhibitor CHIR99021 (MedChemExpress, HY-10182). On day 1, medium was changed to R: RPMI 1640. On day 2, medium was changed to RBA-C59: RPMI 1640 supplemented with 2 mg/mL BSA, 200 μg/mL l-ascorbic acid 2-phosphate (Wako, 321-44823), and 2 µM Wnt-C59 (Biorbyt, orb181132). Medium was then changed every other day starting on day 4 with hiPSC-CM maintenance media RBAI: RPMI 1640 supplemented with 0.5 mg/mL BSA, 200 μg/mL l-ascorbic acid 2-phosphate, and 1 µg/mL E. coli-derived recombinant human insulin. Contracting cells were observed starting on day 7. On day 12-16, hiPSC-CMs were dissociated using DPBS for 20 min at 37 °C, followed by 1:200 Liberase TH (Roche, 5401151001) diluted in DPBS for 20 min at 37 °C. The collected cells were centrifuged at 300 × g for 5 min, filtered through a 100 μm cell strainer (Fisherbrand, 22-363-549), and analyzed by flow cytometry or replated in RBAI media on Matrigel-coated Greiner μClear plates for immunofluorescence.
Neural differentiation
hiPSCs were differentiated into neural progenitor cells by adapting a chemically defined protocol to induce neuroectoderm differentiation from pluripotent stem cells60. Briefly, hiPSCs were split at a 1:10 ratio using 0.5 mM EDTA as above and grown in B8T medium for 1 day. On the first day of differentiation (day 0), medium was changed to neural induction medium containing 12 ng/mL FGF2-G3 (made in-house) and 10 µM SB431542 in B6 medium: BMEM basal medium61, 15 mg/L recombinant human insulin, 10 mg/L recombinant human transferrin, 20 µg/L sodium selenite, 50 mg/L l-ascorbic acid 2-phosphate. Media was changed daily. On day 12, cells were dissociated with TrypLE for 5 min at 37 °C, centrifuged at 400 × g for 5 min, and analyzed by flow cytometry or western blot.
Hepatocyte differentiation
Differentiation into hepatocytes was performed according to our modified version of protocols previously described62. hiPSCs were split with 0.5 mM EDTA as above and grown in B8 medium for 4 days until reaching ~75% confluency. On the first day of differentiation (day 0), medium was changed to DE medium: RPMI 1640 supplemented with 1% B27 minus insulin (v/v, Gibco, A1895601), 100 ng/mL Activin A (Peprotech, 120-14E), and 3 µM CHIR99021. On day 4, medium was changed to HE medium: RPMI 1640 supplemented with 1% B27 minus insulin, 5 ng/mL FGF2-G3 (made in-house), 20 ng/mL BMP4, and 0.5% DMSO (v/v, Fisher, BP231-100). On day 9, medium was changed to IMH medium: RPMI 1640 supplemented with 1% B27 minus insulin, 20 ng/mL HGF (Peprotech, 100-39H), and 0.5% DMSO. Media was changed daily during differentiation at 24 h intervals. On day 10, cells were dissociated with TrypLE for 5 min at 37 °C, centrifuged at 300 × g for 5 min, and analyzed by flow cytometry or immunofluorescence.
hiPSC-derived mammary organoids
hiPSCs were plated (2000 cells/well) in uncoated, 96-well V-bottom plates in B8T containing 0.2% poly(vinyl alcohol) (Thermo Scientific, 396760010) to form spheroids. Differentiation into hiPSC-MEC organoids was initiated one day later (day 0) following the mammary epithelial cell differentiation protocol listed above, with all differentiation media supplemented with 0.2% poly(vinyl alcohol). On day 3, hiPSC-MEC organoids were collected for immunofluorescence, transferred to suspension culture, or embedded in Matrigel. For suspension culture, hiPSC-MEC organoids were transferred to a 24-well ultra-low attachment plate containing M87 media using a P1000 pipette. Matrigel embedded organoids were formed by adapting protocols used to generate primary breast cancer organoids63. Briefly, 5–10 hiPSC-MEC organoids were resuspended in 40 µL cold Matrigel (Corning, 354230) and transferred as a drop onto 1 well of a pre-warmed, 24-well ultra-low attachment plate. Plates were incubated at 37 °C for 30 min, then 800 µL of prewarmed M87 media was gently added to each well and plates were returned to 37 °C incubator. After 5 days of growth in suspension or embedded in Matrigel, hiPSC-MEC organoids were imaged using a Ti-E inverted fluorescent microscope (Nikon Instruments).
Mammosphere formation assay
hiPSC-MECs were dissociated using TrypLE as previously described and single-cell suspensions were visually confirmed using a trypan blue (Gibco, 15250061) cell viability dye. hiPSC-MECs were plated at a low seeding density (1000 cells/cm2) in 24-well ultra-low attachment plates (Corning, 3473) in mammosphere media containing 2 mM l-glutamine (Sigma, G3126), 20 ng/mL EGF, 10 ng/mL FGF2-G3 (made in house53), 1x B27 supplement (Gibco, 17504044), and 5 µM Y27632 (MedChemExpress, HY10071) in DMEM/F12. Cells were incubated at 37 °C undisturbed for 7 days. Entire wells were imagined as 5 × 5 stitched phase contrast images using a Ti-E inverted microscope. The number and diameter of spheres ≥70 µm per well were quantified using Fiji (ImageJ) software64. Mammosphere formation efficiency is defined as the number of spheres ≥70 µM divided by the number of cells plated.
hiPSC-MEC proliferation assays
hiPSC-MEC proliferation was assessed with a resazurin-based cell viability assay or a population doubling level assessment. hiPSC-MECs grown in indicated culture conditions were assessed with a resazurin-based viability assay as previously described61. Briefly, media was removed from cells and replaced with 20 µg/mL resazurin (Thermo Scientific, B21187.06) in DPBS. Plates were incubated at 37 °C for 2.5 h and read on a Varioskan LUX multimode plate reader (Thermo Scientific) in fluorescent mode using top read, an excitation wavelength of 560 nm, and an emission wavelength of 590 nm.
Population doubling level (PDL) of hiPSC-MECs over 3 passages was calculated from cell number counts (ViCell, Beckman Coulter) according to the following formula: PDL = 3.32[log10(n/n0)], where n = cell number and n0 = number of cells seeded.
Drug viability assay
Olaparib (LC Laboratories, O-9201) and talazoparib (MedChemExpress, HY-16106) were resuspended to 10 mM in DMSO and aliquots were stored at −80 °C. Doxorubicin (LC Laboratories, D-4000) was resuspended to 10 mM in cell culture-grade water (Corning) and aliquots were stored at −20 °C.
hiPSC-MECs were dissociated in TrypLE and plated at 2000 cells/well on collagen I-coated 384-well plates in M87 + T media. The following day, cells were treated for the indicated duration with olaparib (0.001–100 μM; 6 days), talazoparib (0.0001–10 μM; 6 days), or doxorubicin (0.0001–10 μM, 3 days) diluted in M87 + T media. 10 μM staurosporine (MedChemExpress, HY-15141) and 0.1% DMSO (for olaparib and talazoparib) or water (for doxorubicin) were used as positive and negative controls, respectively. Cell viability following drug treatment was assessed using a resazurin-based assay as previously described and reiterated above (see “hiPSC-MEC proliferation assays”)61.
DNA damage and repair assessment
hiPSC-MECs were dissociated with TrypLE and plated at 5000 cells/well on collagen I-coated 96-well plates in M87 + T media and allowed to proliferate for 2 days. Cells were then treated for 48 h with olaparib (1 or 10 μM), talazoparib (0.1 or 1 μM), or DMSO (0.1%) diluted in M87 + T. Cells were fixed, permeabilized, and immunostained for γH2AX and RAD51 (see Supplementary Data 3 for antibody details). At least 4 representative images per condition were collected using a Ti-E inverted fluorescent microscope. Images were analyzed by Fiji (ImageJ) software64 using a nuclear mask to quantify mean nuclear fluorescence intensity.
Immunofluorescence
Cells were fixed with 4% paraformaldehyde in DPBS for 15 min, washed for 3 ×5 min with DPBS, permeabilized with 0.3% Triton X-100 (Fisher, BP151-100) in DPBS for 10 min (except for staining cell surface markers), washed for 3 ×5 min with DPBS, and blocked with 1% BSA in DPBS for 1 h (all at RT). Cells were then stained overnight at 4 °C with primary antibody (see Supplementary Data 3 for antibody details) diluted in staining buffer: 1% BSA and 0.1% Tween 20 (Fisher, BP337-100) in DPBS. The next day, cells were blocked with 10% BSA in DPBS for 10 min at RT and then stained with secondary antibody diluted in staining buffer for 2 h at RT in the dark. Cells were washed for 3 ×10 min with 0.1% Tween 20 in DPBS, with NucBlue (Invitrogen, R37606) added during the first wash. Cells were imaged with a Ti-E inverted fluorescent microscope and a Zyla sCMOS camera (Andor) using NIS-Elements AR software. Secondary-only wells were used to establish exposure time.
For organoid immunofluorescence, all steps were performed in 1.5 mL microcentrifuge tubes on a shaker, using sedimentation to separate organoids before changing media. hiPSC-MEC organoids were fixed with 4% paraformaldehyde in DPBS for 60 min at 4 °C, washed for 3 ×5 min with DPBS, permeabilized with 0.3% Triton X-100 in DPBS for 30 min (except for staining cell surface markers), washed for 3 ×10 min with DPBS, and blocked with 10% BSA in DPBS for 2 h (at RT unless otherwise specified). Cells were then stained overnight at 4 °C with primary antibody diluted in staining buffer. The next day, cells were washed for 3 ×20 min with DPBS and then stained overnight at 4 °C with secondary antibody diluted in staining buffer. The next day, cells were washed for 3 ×20 min with 0.1% Tween 20 in DPBS, with NucBlue added during the first wash. Organoids were then transferred to microscope slides overlaid with glass coverslips and imaged as z-stacks with a Nikon W1 dual cam spinning disk confocal microscope (Nikon Instruments).
Flow cytometry
Dissociated cells were fixed with 4% paraformaldehyde (Electron Microscopy Services, 15713-S) in DPBS for 10 min at RT and washed with staining buffer: 5% CCS and 0.1% sodium azide (Sigma, S2002) in DPBS. Cells were then permeabilized in DPBS with 0.1% saponin (Sigma, 47036) for 15 min at RT and washed in staining buffer with 0.1% saponin. Once fixed and permeabilized, cells were stained with conjugated antibody or isotype control (see Supplementary Data 3 for antibody details) in staining buffer with 0.1% saponin. Cells were washed 3× in staining buffer with saponin prior to data collection. All data were collected using a CytoFLEX flow cytometer (Beckman Coulter) and analyzed with CytExpert (Beckman Coulter) and FlowJo (FlowJo LLC) software using isotype controls to set positive gates.
Quantitative real-time PCR
Cell lysates for RT-qPCR were collected in 300 μL TRIzol (Invitrogen, 15596026) and total RNA was isolated using the Direct-zol RNA Microprep Kit (Zymo Research, R2062) following the manufacturer’s protocol. Reverse transcription was performed from 1 μg of RNA using Maxima H Minus cDNA Synthesis Master Mix (Thermo Scientific, M1662) following the manufacturer’s protocol, and the resultant cDNA was diluted 1:10. RT-qPCR was performed using TaqMan gene expression assays (see Supplementary Data 3 for TaqMan probes) and TaqMan Fast Advanced Master Mix (Applied Biosystems, 4444964) following the manufacturer’s protocol. All PCR reactions were prepared in triplicate in a 384-well format. Data were collected using the QuantStudio 5 Real-Time PCR system. Relative quantification of gene expression was calculated using the 2-DDCt method65, with normalization to reference gene(s) (18S and GAPDH) and a control sample (undifferentiated hiPSCs; ISO hiPSCs).
Western blot
Cells were washed and harvested in cold DPBS and collected by centrifugation at 20,000 × g for 5 min at 4 °C. The cell pellet was lysed in RIPA lysis buffer (Thermo Scientific, 89900) containing Halt protease and phosphatase inhibitors (Thermo Scientific, 78440) and sonicated (2 pulses of 15 s at 50% amplitude) to shear DNA. The samples were centrifuged at 20,000 × g for 15 min at 4 °C and concentration of protein in the supernatant was determined using a BCA Protein Assay (Thermo Scientific, 23227) following manufacturer’s instructions. Total protein (25 µg) from each sample was denatured with NuPAGE LDS sample buffer (Invitrogen, NP0008) containing Bolt sample reducing agent (Invitrogen, B0009) and boiled at 100 °C for 5 min. Proteins were separated by gel electrophoresis using NuPAGE Novex 3-8% Tris-Acetate precast gels (Invitrogen, EA0375BOX) and transferred onto a nitrocellulose membrane using the iBlot Gel Transfer Device (Invitrogen). Membranes were blocked in DPBS containing 1% non-fat milk (Sigma, 1153630500) and 0.05% Tween 20 for 1 h at RT and then incubated at 4 °C overnight with primary antibody (see Supplementary Data 3 for antibody details). Following washes, membranes were incubated with the appropriate secondary antibody for 1 h at RT. Membranes were imaged using an Odyssey IR Imaging System (LI-COR) and immunoreactive bands were visualized using Fiji (ImageJ) software64.
CRISPR/Cas9-mediated knockout
The Alt-R CRISPR-Cas9 System (Integrated DNA Technologies) was used to generate a BRCA1 KO hiPSC line. Custom, guide-specific CRISPR RNA (crRNA) oligonucleotides targeting BRCA1 were designed using Benchling and CRISPOR design tools (see Supplementary Data 3 for crRNA sequences). crRNA (IDT) and tracrRNA (IDT) were reconstituted in nuclease free duplex buffer (IDT, 11-01-03-01) to a concentration of 200 µM. crRNA and tracrRNA were combined 1:1 (final duplex concentration of 100 µM) and heated at 95 °C for 5 min. After allowing it to cool to RT, the duplex was combined with Alt-R S.p. HiFi Cas9 Nuclease V3 (IDT, 1081061) to achieve a molarity ratio of 1:2 Cas9:Duplex in the final volume. The Cas9 and RNA duplex were incubated at RT for 20 min to allow the formation of the RNP complex.
hiPSCs from a control cell line (GW143c2) were dissociated in TrypLE for 3 min at RT. 1 × 106 cells were electroporated with the RNP complex prepared above and 4 µM Alt-R Electroporation Enhancer (IDT, 1075916) using a Neon Transfection System (Invitrogen, MPK10096). To screen specific crRNAs for efficacy, pooled cells were collected 4 days after transfection for DNA extraction and Sanger sequencing to confirm cutting. To establish KO lines, transfected cells were seeded at a low density (ranging from 1:6 to 1:48 in wells of 6-well plates) in B8T media, and individual clones were picked manually 3–5 days after transfection for expansion. Clones were screened by Sanger sequencing at the following passage to identify candidate KOs.
CRISPR/Cas9-mediated homology-directed repair
The Alt-R CRISPR-Cas9 System (Integrated DNA Technologies) was used to correct the BRCA1 c.68_69delAG variant in the patient-derived BC8-1 hiPSC line and knockin the BRCA1 c.68_69delAG variant in the GW143c2 control hiPSC line. crRNAs targeting regions adjacent to the desired edit site were designed using Benchling and CRISPOR design tools and used to prepare RNP complexes as described above. Correction and knock-in specific repair templates were designed as 90 nucleotide sequences flanking the desired edit and included silent mutations to prevent further cutting. See Supplementary Data 3 for crRNA and HDR template sequences.
hiPSCs were dissociated in TrypLE for 3 min at RT. 1 × 106 cells were electroporated with the RNP complex, 4 µM Alt-R Electroporation Enhancer, and 4 µM repair template (IDT) using a Neon Transfection System. Transfected cells were seeded at a low density (ranging from 1:2 to 1:48 in wells of 6-well plates) in B8T media containing 0.5 µM Alt-R HDR Enhancer V2 (IDT, 10007921) for the first 24 h. Individual clones were picked manually 3–5 days after transfection for expansion. Clones were screened by Sanger sequencing at the following passage to identify candidate correction and knockin clones.
Sanger sequencing
gDNA was extracted from cells using QuickExtract DNA Extraction Solution (Lucigen, QE09050) according to manufacturer’s protocol. DNA purity and quantity were assessed using a Thermo Scientific NanoDrop 8000. The targeted region was amplified by PCR using the Q5 High-Fidelity 2x Master Mix (New England Biosciences, M0492L) according to manufacturer’s protocol and sequenced by Sanger sequencing (Eurofins Genomics). See Supplementary Data 3 for PCR primers.
RNA-sequencing
RNA-sequencing was performed on at least 3 cell lines from the following cell types: primary breast cancer cells, undifferentiated hiPSCs, hiPSC-derived mammary epithelial cells (hiPSC-MEC), hiPSC-derived endothelial cells (hiPSC-EC), and hiPSC-derived cardiomyocytes (hiPSC-CM). Cell lysates were collected in 300 μL TRIzol and total RNA was isolated using the Direct-zol RNA Microprep Kit, following the manufacturer’s protocol including on-column DNase digestion to remove genomic DNA. The purity and quantity of RNA were assessed using a Thermo Scientific NanoDrop 8000 and samples were shipped to BGI on dry ice for further QC, library preparation, and sequencing using BGI’s DNBSEQ platform. ~40 million paired-end (100 bp) reads were generated for each sample. Reads were mapped to the GRCh38 human reference genome using Subread software66. Gene expression levels were estimated using featureCounts function in the Subread software67. PCA plots were generated in R.
Whole genome sequencing
Whole genome sequencing was performed on at least one BC-hiPSC line derived from each of the 10 breast tumor samples (2 hiPSC lines from BC6; 3 hiPSC lines from BC8) and patient-matched healthy tissue when available (BC2, BC6, BC7, BC8, and BC9). Genomic DNA was extracted using a Quick-DNA Miniprep Plus Kit (Zymo Research, D4069), following the manufacturer’s protocol including Proteinase K digestion. The purity and quantity of DNA were assessed using a Thermo Scientific NanoDrop 8000 and samples were shipped to BGI on dry ice for further quality control, library preparation, and paired-end (150 bp) sequencing using BGI’s DNBSEQ platform. Raw reads were processed using SOAPnuke to trim/remove adaptors, low-quality reads, and N reads68. Clean sequencing reads were aligned to the GRCh38 human reference genome using the Burrows’ Wheeler Aligner (BWA-MEM)69. Variant calling followed best practice workflows outlined by the Genome Analysis Toolkit (GATK)70. Genomic variants, including SNVs and InDels, were called using GATK Haplotype Caller. Joint genotyping and variant filtration were performed using the variant quality score recalibration (VQSR) method. Variants were then annotated using ANNOVAR, including annotations for refGene, allele frequencies from gnomAD, and functional prediction tools (SIFT, Polyphen 2, MutationTaster, CADD)71.
We selected SNVs and InDels as candidate variants (Supplementary Data 2) if they satisfied the following criteria: (1) Variant occurs within a custom list of 212 genes (Supplementary Data 4), including genes with recurrent somatic mutations in breast cancer and germline, breast cancer susceptibility genes25. (2) Variant located in exonic or splicing region. (3) Variant classified as nonsynonymous, nonsense, or frameshift variant. (4) For nonsynonymous variants, predicted to alter protein function in at least two of four functional prediction tools: SIFT (SIFT_pred = “D”/deleterious), Polyphen 2 (Polyphen2_pred = “D”/probably damaging or “P”/possibly damaging), MutationTaster (MutationTaster_pred = “D”/disease_causing or “A”/disease_causing_automatic), CADD (CADD_phred ≥ 15). Only variant filtering steps (1) and (2) were applied when identifying somatic variants unique to individual BC-hiPSC lines (Fig. 1I, J; Supplementary Fig. 1E, F).
Statistical analysis
Data were analyzed in Excel and GraphPad Prism. Detailed statistical information is included in the corresponding figure legends. Data were presented as mean ± SEM. Data were checked for normal distribution and comparisons were conducted via an unpaired two-tailed Student’s t-test or one-way analysis of variance (ANOVA) with Dunnett’s or Tukey’s multiple comparisons test. Significant differences are defined as P < 0.05 (*), P < 0.01 (**), P < 0.005 (***), and P < 0.0001 (****). No statistical methods were used to predetermine sample size. The experiments were not randomized, and the investigators were not blinded to allocation during experiments and outcome assessment.
Supplementary information
Acknowledgements
This work was supported by funding from the National Institutes of Health R01 CA261898 (P.W.B.), F31 CA247395 (C.J.W.), and T32 CA009560 (C.J.W.) as well as the Leducq Foundation (P.W.B.). Patient recruitment and tissue procurement was completed by the Northwestern University Pathology Core Facility, which is supported by a Cancer Center Support Grant (NCI CA060553).
Author contributions
P.W.B. and C.J.W. designed the research. C.J.W. completed most experiments, along with N.U., P.P., and R.B.C. WGS-seq data was analyzed by M.B. P.W.B. supervised the project. C.J.W. wrote the initial manuscript, and all authors read and approved the final manuscript.
Data availability
RNA-sequencing data that support the findings of this study have been deposited in the NCBI Gene Expression Omnibus with the primary accession code GSE272395.
Material availability
hiPSC lines used in this study are available from the lead contact, Paul Burridge (paul.burridge@northwestern.edu), with a completed Materials Transfer Agreement.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41698-025-00837-5.
References
- 1.Papapetrou, E. P. Patient-derived induced pluripotent stem cells in cancer research and precision oncology. Nat. Med22, 1392–1401 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wesely, J. et al. Acute myeloid leukemia iPSCs reveal a role for RUNX1 in the maintenance of human leukemia stem cells. Cell Rep.31, 107688 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ye, Z. et al. Differential sensitivity to JAK inhibitory drugs by isogenic human erythroblasts and hematopoietic progenitors generated from patient-specific induced pluripotent stem cells. Stem Cells32, 269–278 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang, C. J. et al. Dissecting the contributions of cooperating gene mutations to cancer phenotypes and drug responses with patient-derived iPSCs. Stem Cell Rep.10, 1610–1624 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu, K. et al. Efficient generation of transgene-free induced pluripotent stem cells from normal and neoplastic bone marrow and cord blood mononuclear cells. Blood117, e109–e119 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kotini, A. G. et al. Functional analysis of a chromosomal deletion associated with myelodysplastic syndromes using isogenic human induced pluripotent stem cells. Nat. Biotechnol.33, 646–655 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumano, K. et al. Generation of induced pluripotent stem cells from primary chronic myelogenous leukemia patient samples. Blood119, 6234–6242 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Chao, M. P. et al. Human AML-iPSCs reacquire leukemic properties after differentiation and model clonal variation of disease. Cell Stem Cell20, 329–344.e327 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gandre-Babbe, S. et al. Patient-derived induced pluripotent stem cells recapitulate hematopoietic abnormalities of juvenile myelomonocytic leukemia. Blood121, 4925–4929 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kotini, A. G. et al. Patient-derived iPSCs faithfully represent the genetic diversity and cellular architecture of human acute myeloid leukemia. Blood Cancer Discov.4, 318–335 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kotini, A. G. et al. Stage-specific human induced pluripotent stem cells map the progression of myeloid transformation to transplantable leukemia. Cell Stem Cell20, 315–328.e317 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bedel, A. et al. Variable behavior of iPSCs derived from CML patients for response to TKI and hematopoietic differentiation. PLoS One8, e71596 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye, Z. et al. Human-induced pluripotent stem cells from blood cells of healthy donors and patients with acquired blood disorders. Blood114, 5473–5480 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hosoi, M. et al. Generation of induced pluripotent stem cells derived from primary and secondary myelofibrosis patient samples. Exp. Hematol.42, 816–825 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Perou, C. M. et al. Molecular portraits of human breast tumours. Nature406, 747–752 (2000). [DOI] [PubMed] [Google Scholar]
- 16.Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature490, 61–70 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Litton, J. K. et al. Talazoparib in patients with advanced breast cancer and a germline BRCA Mutation. N. Engl. J. Med.379, 753–763 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robson, M. et al. Olaparib for metastatic breast cancer in patients with a germline BRCA Mutation. N. Engl. J. Med.377, 523–533 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Tutt, A. N. J. et al. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N. Engl. J. Med.384, 2394–2405 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dias, M. P., Moser, S. C., Ganesan, S. & Jonkers, J. Understanding and overcoming resistance to PARP inhibitors in cancer therapy. Nat. Rev. Clin. Oncol.18, 773–791 (2021). [DOI] [PubMed] [Google Scholar]
- 21.Vargo-Gogola, T. & Rosen, J. M. Modelling breast cancer: one size does not fit all. Nat. Rev. Cancer7, 659–672 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Ben-David, U. et al. Genetic and transcriptional evolution alters cancer cell line drug response. Nature560, 325–330 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeRose, Y. S. et al. Patient-derived models of human breast cancer: protocols for in vitro and in vivo applications in tumor biology and translational medicine. Curr. Protoc. Pharm.14, 14–23 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell131, 861–872 (2007). [DOI] [PubMed] [Google Scholar]
- 25.Pereira, B. et al. The somatic mutation profiles of 2433 breast cancers refines their genomic and transcriptomic landscapes. Nat. Commun.7, 11479 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Papp, B. & Plath, K. Epigenetics of reprogramming to induced pluripotency. Cell152, 1324–1343 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molyneux, G. et al. BRCA1 basal-like breast cancers originate from luminal epithelial progenitors and not from basal stem cells. Cell Stem Cell7, 403–417 (2010). [DOI] [PubMed] [Google Scholar]
- 28.Polyak, K. Breast cancer: origins and evolution. J. Clin. Investig.117, 3155–3163 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li, L. et al. TFAP2C- and p63-dependent networks sequentially rearrange chromatin landscapes to drive human epidermal lineage commitment. Cell Stem Cell24, 271–284.e278 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farmer, H. et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature434, 917–921 (2005). [DOI] [PubMed] [Google Scholar]
- 31.Bryant, H. E. et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature434, 913–917 (2005). [DOI] [PubMed] [Google Scholar]
- 32.Yndestad, S. et al. Homologous recombination deficiency across subtypes of primary breast cancer. JCO Precis Oncol.7, e2300338 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Struewing, J. P. et al. The carrier frequency of the BRCA1 185delAG mutation is approximately 1 percent in Ashkenazi Jewish individuals. Nat. Genet.11, 198–200 (1995). [DOI] [PubMed] [Google Scholar]
- 34.Kim, J. et al. An iPSC line from human pancreatic ductal adenocarcinoma undergoes early to invasive stages of pancreatic cancer progression. Cell Rep.3, 2088–2099 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raya, A. et al. Disease-corrected haematopoietic progenitors from Fanconi anaemia induced pluripotent stem cells. Nature460, 53–59 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li, H. et al. The Ink4/Arf locus is a barrier for iPS cell reprogramming. Nature460, 1136–1139 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawamura, T. et al. Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature460, 1140–1144 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muller, L. U. et al. Overcoming reprogramming resistance of Fanconi anemia cells. Blood119, 5449–5457 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marion, R. M. et al. A p53-mediated DNA damage response limits reprogramming to ensure iPS cell genomic integrity. Nature460, 1149–1153 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Minussi, D. C. et al. Breast tumours maintain a reservoir of subclonal diversity during expansion. Nature592, 302–308 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim, C. et al. Chemoresistance evolution in triple-negative breast cancer delineated by single-cell sequencing. Cell173, 879–893.e813 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keller, P. J. et al. Mapping the cellular and molecular heterogeneity of normal and malignant breast tissues and cultured cell lines. Breast Cancer Res.12, R87 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee, J. K. et al. Different culture media modulate growth, heterogeneity, and senescence in human mammary epithelial cell cultures. PLoS One13, e0204645 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drost, R. et al. BRCA1185delAG tumors may acquire therapy resistance through expression of RING-less BRCA1. J. Clin. Investig.126, 2903–2918 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harvey-Jones, E. et al. Longitudinal profiling identifies co-occurring BRCA1/2 reversions, TP53BP1, RIF1 and PAXIP1 mutations in PARP inhibitor-resistant advanced breast cancer. Ann. Oncol.35, 364–380 (2024). [DOI] [PubMed] [Google Scholar]
- 46.Lord, C. J. & Ashworth, A. PARP inhibitors: synthetic lethality in the clinic. Science355, 1152–1158 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shah, J. B. et al. Analysis of matched primary and recurrent BRCA1/2 mutation-associated tumors identifies recurrence-specific drivers. Nat. Commun.13, 6728 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pettitt, S. J. et al. Clinical BRCA1/2 reversion analysis identifies hotspot mutations and predicted neoantigens associated with therapy resistance. Cancer Discov.10, 1475–1488 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tobalina, L., Armenia, J., Irving, E., O’Connor, M. J. & Forment, J. V. A meta-analysis of reversion mutations in BRCA genes identifies signatures of DNA end-joining repair mechanisms driving therapy resistance. Ann. Oncol.32, 103–112 (2021). [DOI] [PubMed] [Google Scholar]
- 50.Norquist, B. et al. Secondary somatic mutations restoring BRCA1/2 predict chemotherapy resistance in hereditary ovarian carcinomas. J. Clin. Oncol.29, 3008–3015 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang, Y. et al. Clonal evolution in breast cancer revealed by single nucleus genome sequencing. Nature512, 155–160 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fetterman, K. A. et al. Independent compartmentalization of functional, metabolic, and transcriptional maturation of hiPSC-derived cardiomyocytes. Cell Rep.43, 114160 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuo, H. H. et al. Negligible-cost and weekend-free chemically defined human iPSC culture. Stem Cell Rep.14, 256–270 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fonoudi, H., Lyra-Leite, D. M., Javed, H. A. & Burridge, P. W. Generating a cost-effective, weekend-free chemically defined human induced pluripotent stem cell (hiPSC) culture medium. Curr. Protoc. Stem Cell Biol.53, e110 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lyra-Leite, D. M., Fonoudi, H., Gharib, M. & Burridge, P. W. An updated protocol for the cost-effective and weekend-free culture of human induced pluripotent stem cells. STAR Protoc.2, 100213 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li, L. et al. Ectodermal progenitors derived from epiblast stem cells by inhibition of Nodal signaling. J. Mol. Cell Biol.7, 455–465 (2015). [DOI] [PubMed] [Google Scholar]
- 57.Tchieu, J. et al. A modular platform for differentiation of human PSCs into all major ectodermal lineages. Cell Stem Cell21, 399–410.e397 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qu, Y. et al. Transcriptome and proteome characterization of surface ectoderm cells differentiated from human iPSCs. Sci. Rep.6, 32007 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Magdy, T. et al. Identification of drug transporter genomic variants and inhibitors that protect against doxorubicin-induced cardiotoxicity. Circulation145, 279–294 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheung, C., Bernardo, A. S., Trotter, M. W., Pedersen, R. A. & Sinha, S. Generation of human vascular smooth muscle subtypes provides insight into embryological origin-dependent disease susceptibility. Nat. Biotechnol.30, 165–173 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lyra-Leite, D. M. et al. Nutritional requirements of human induced pluripotent stem cells. Stem Cell Rep.18, 1371–1387 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peters, D. T. et al. Asialoglycoprotein receptor 1 is a specific cell-surface marker for isolating hepatocytes derived from human pluripotent stem cells. Development143, 1475–1481 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sachs, N. et al. A living biobank of breast cancer organoids captures disease heterogeneity. Cell172, 373–386.e310 (2018). [DOI] [PubMed] [Google Scholar]
- 64.Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods9, 676–682 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods25, 402–408 (2001). [DOI] [PubMed] [Google Scholar]
- 66.Liao, Y., Smyth, G. K. & Shi, W. The Subread aligner: fast, accurate and scalable read mapping by seed-and-vote. Nucleic Acids Res.41, e108 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics30, 923–930 (2014). [DOI] [PubMed] [Google Scholar]
- 68.Chen, Y. et al. SOAPnuke: a MapReduce acceleration-supported software for integrated quality control and preprocessing of high-throughput sequencing data. Gigascience7, 1–6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics25, 1754–1760 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Auwera, G. V. D. & O’Connor, B. D. Genomics in the Cloud: Using Docker, GATK, and WDL in Terra. First edition (O’Reilly Media, 2020).
- 71.Wang, K., Li, M. & Hakonarson, H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res.38, e164 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA-sequencing data that support the findings of this study have been deposited in the NCBI Gene Expression Omnibus with the primary accession code GSE272395.
hiPSC lines used in this study are available from the lead contact, Paul Burridge (paul.burridge@northwestern.edu), with a completed Materials Transfer Agreement.