Abstract
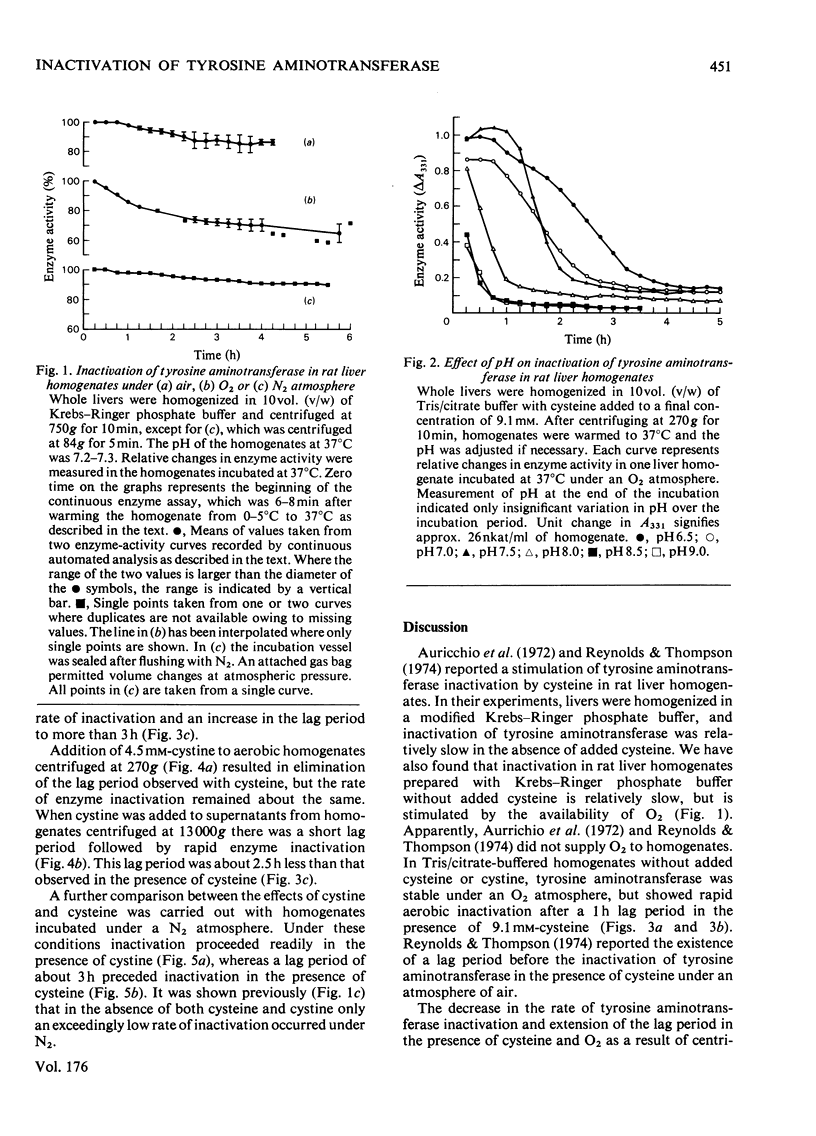
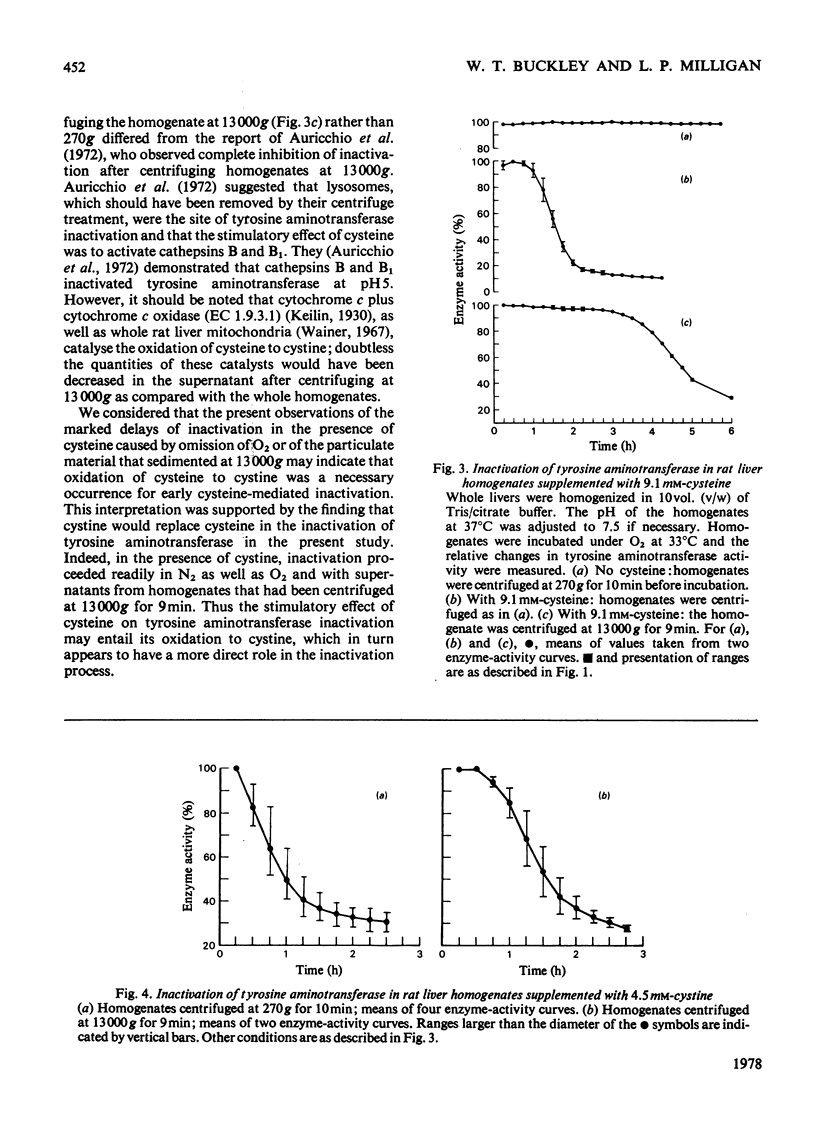
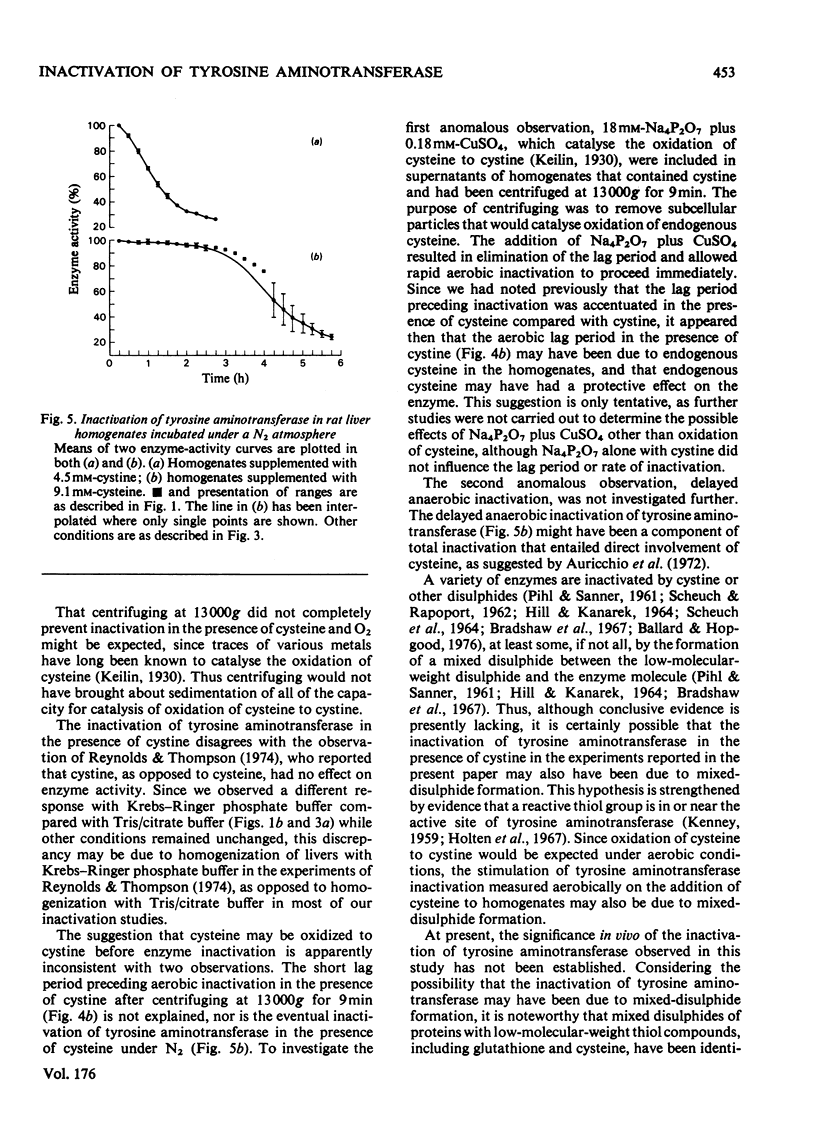
1. Inactivation of tyrosine aminotransferase was studied in rat liver homogenates. Under an O2 atmosphere with cysteine added, inactivation was rapid after a lag period of approx. 1h, whereas a N2 atmosphere extended the lag period to approx. 3h. 2. Replacement of cysteine with cystine resulted in rapid inactivation both aerobically and anaerobically. 3. Removal of the particulate fraction by centrifuging rat liver homogenates at 13,000g for 9min resulted in an aerobic lag period of 0.5h in the presence of cystine and approx. 3h in the presence of cysteine. 4. It is proposed that the stimulatory effect of cysteine on tyrosine aminotransferase inactivation occurs largely as a result of oxidation to cystine, which appears to be a more directly effective agent.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auricchio F., Mollica L., Liguori A. Inactivation of tyrosine aminotransferase in neutral homogenates and rat liver slices. Biochem J. 1972 Oct;129(5):1131–1138. doi: 10.1042/bj1291131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard F. J., Hopgood M. F. Inactivation of phosphoenolypyruvate carboxykinase (GTP) by liver extracts. Biochem J. 1976 Mar 15;154(3):717–724. doi: 10.1042/bj1540717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beneking M., Schmidt H., Weiss G. Subcellular distribution of a factor inactivating tyrosine aminotransferase. Study of its mechanism and relationship to different forms of the enzyme. Eur J Biochem. 1978 Jan 2;82(1):235–243. doi: 10.1111/j.1432-1033.1978.tb12016.x. [DOI] [PubMed] [Google Scholar]
- Bradshaw R. A., Kanarek L., Hill R. L. The preparation, properties, and reactivation of the mixed disulfide derivative of egg white lysozyme and L-cystine. J Biol Chem. 1967 Sep 10;242(17):3789–3798. [PubMed] [Google Scholar]
- HILL R. L., KANAREK L. THE SUBUNITS OF FUMARASE. Brookhaven Symp Biol. 1964 Dec;17:80–97. [PubMed] [Google Scholar]
- Hannah R., Sahib M. K. Stabilization of rat liver tyrosine aminotransferase by tetracycline. Biochem J. 1975 Sep;150(3):329–333. doi: 10.1042/bj1500329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrap K. R., Jackson R. C., Riches P. G., Smith C. A., Hill B. T. The occurrence of protein-bound mixed disulfides in rat tissues. Biochim Biophys Acta. 1973 May 17;310(1):104–110. doi: 10.1016/0005-2795(73)90012-3. [DOI] [PubMed] [Google Scholar]
- Hayashi S. I., Granner D. K., Tomkins G. M. Tyrosine aminotransferase. Purificaton and characterization. J Biol Chem. 1967 Sep 25;242(18):3998–4006. [PubMed] [Google Scholar]
- Holten D., Wicks W. D., Kenney F. T. Studies on the role of vitamin B6 derivatives in regulating tyrosine alpha-ketoglutarate transaminase activity in vitro and in vivo. J Biol Chem. 1967 Mar 10;242(5):1053–1059. [PubMed] [Google Scholar]
- KENNEY F. T. Properties of partially purified tyrosine-alpha-ketoglutarate transaminase from rat liver. J Biol Chem. 1959 Oct;234:2707–2712. [PubMed] [Google Scholar]
- Modig H. Cellular mixed disulphides between thiols and proteins, and their possible implication for radiation protection. Biochem Pharmacol. 1968 Feb;17(2):177–186. doi: 10.1016/0006-2952(68)90321-3. [DOI] [PubMed] [Google Scholar]
- Reynolds R. D., Thompson S. D. Irreversible inactivation of rat liver tyrosine aminotransferase. Arch Biochem Biophys. 1974 Sep;164(1):43–51. doi: 10.1016/0003-9861(74)90006-x. [DOI] [PubMed] [Google Scholar]
- Rodriguez J. M., Pitot H. C. Studies on the conversion of multiple forms of tyrosine aminotransferase in rat liver. Arch Biochem Biophys. 1976 Nov;177(1):185–195. doi: 10.1016/0003-9861(76)90428-8. [DOI] [PubMed] [Google Scholar]
- SCHEUCH D., RAPOPORT S. [The behavior of inorganic pyrophosphatase, triosephosphate dehydrogenase and glucose-6-phosphate dehydrogenase in oxidation of their essential SH groups]. Acta Biol Med Ger. 1962;8:31–41. [PubMed] [Google Scholar]
- Seubert W., Hamm H. H. Inactivation and ATP-dependent reactivation of tyrosine aminotransferase in vitro by membrane bound enzymes from rat liver and kidney cortex. Biochem Biophys Res Commun. 1975 Jul 8;65(1):1–7. doi: 10.1016/s0006-291x(75)80053-2. [DOI] [PubMed] [Google Scholar]
- Wainer A. Mitochondrial oxidation of cysteine. Biochim Biophys Acta. 1967 Aug 29;141(3):466–472. doi: 10.1016/0304-4165(67)90174-2. [DOI] [PubMed] [Google Scholar]


