Abstract
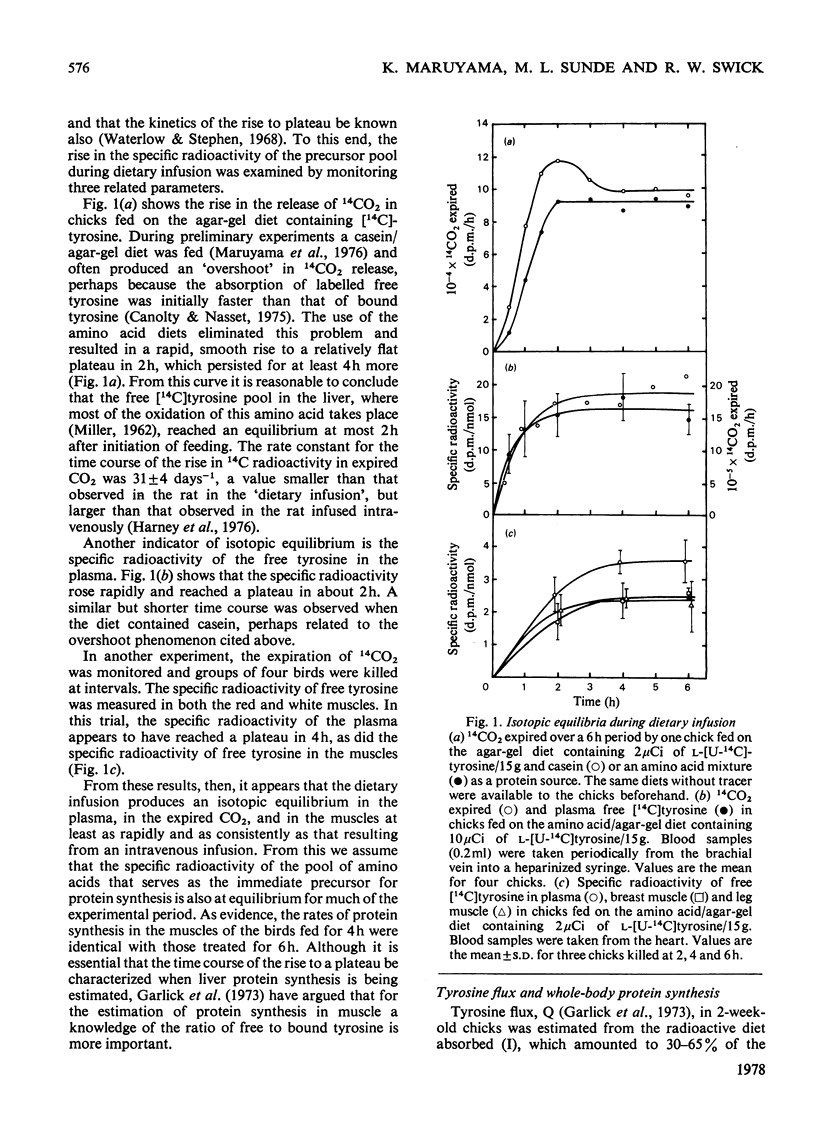
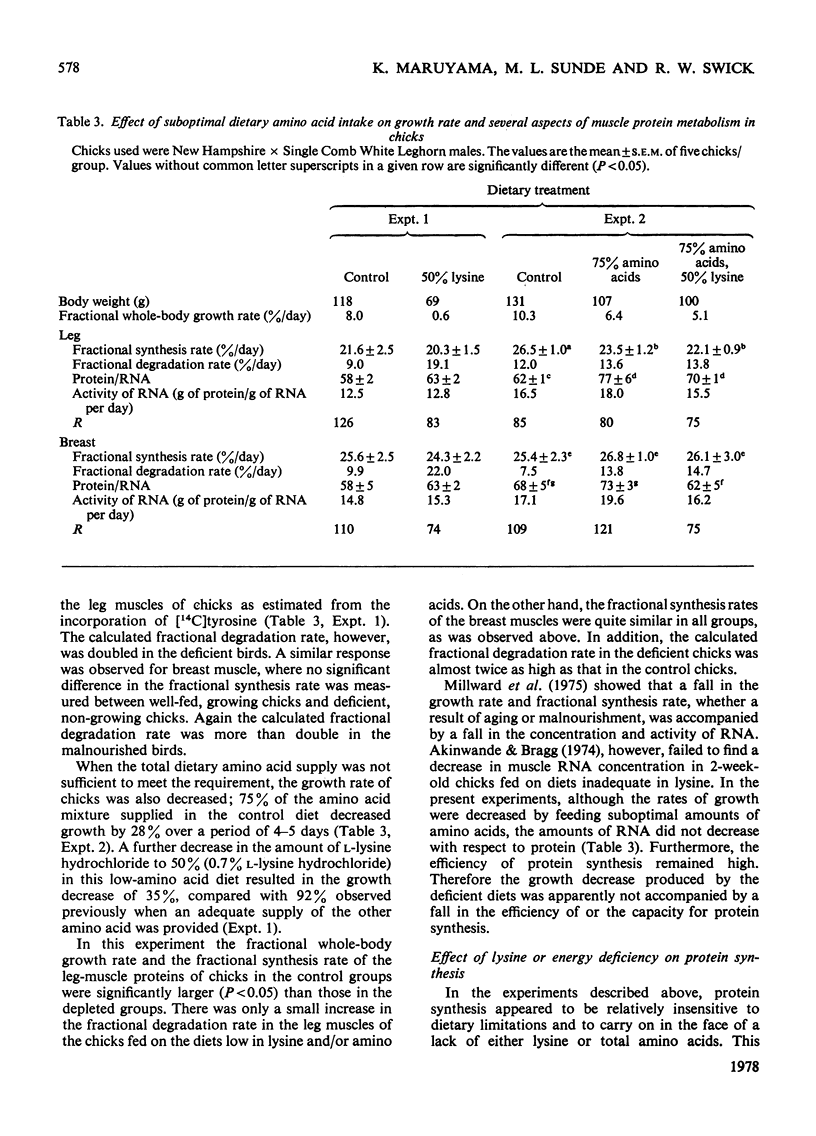
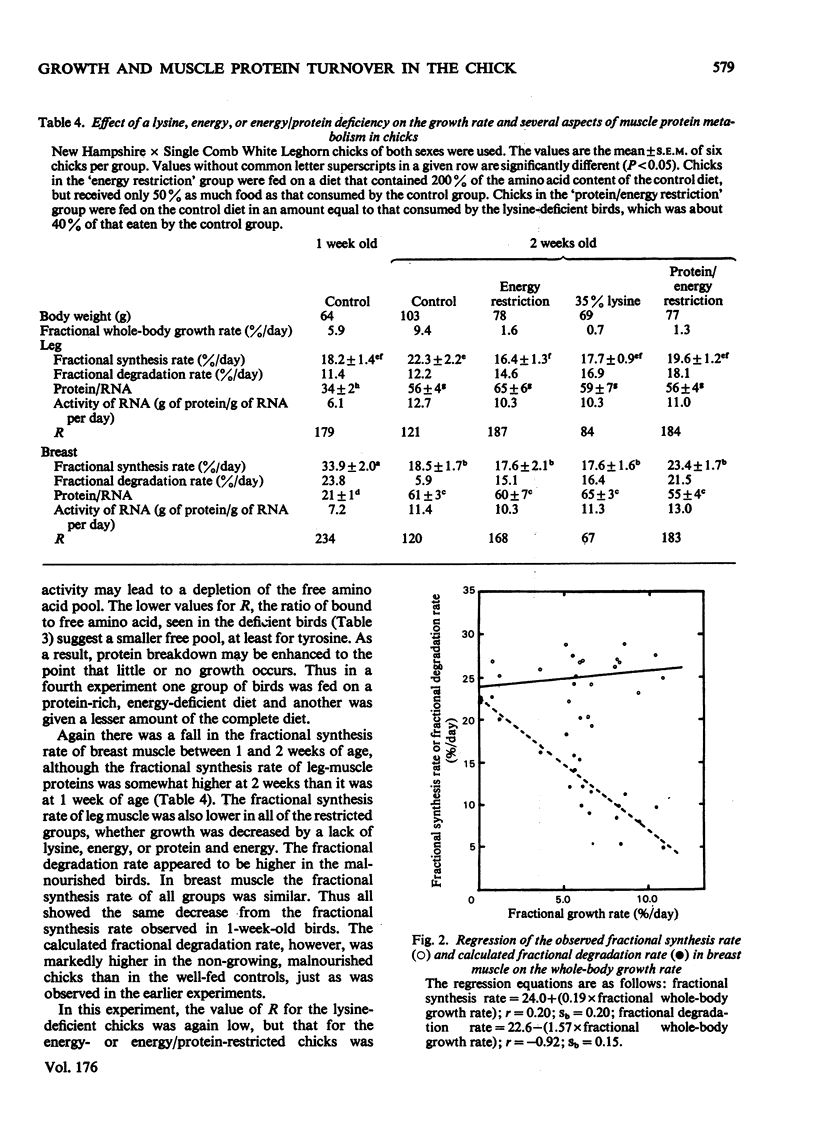
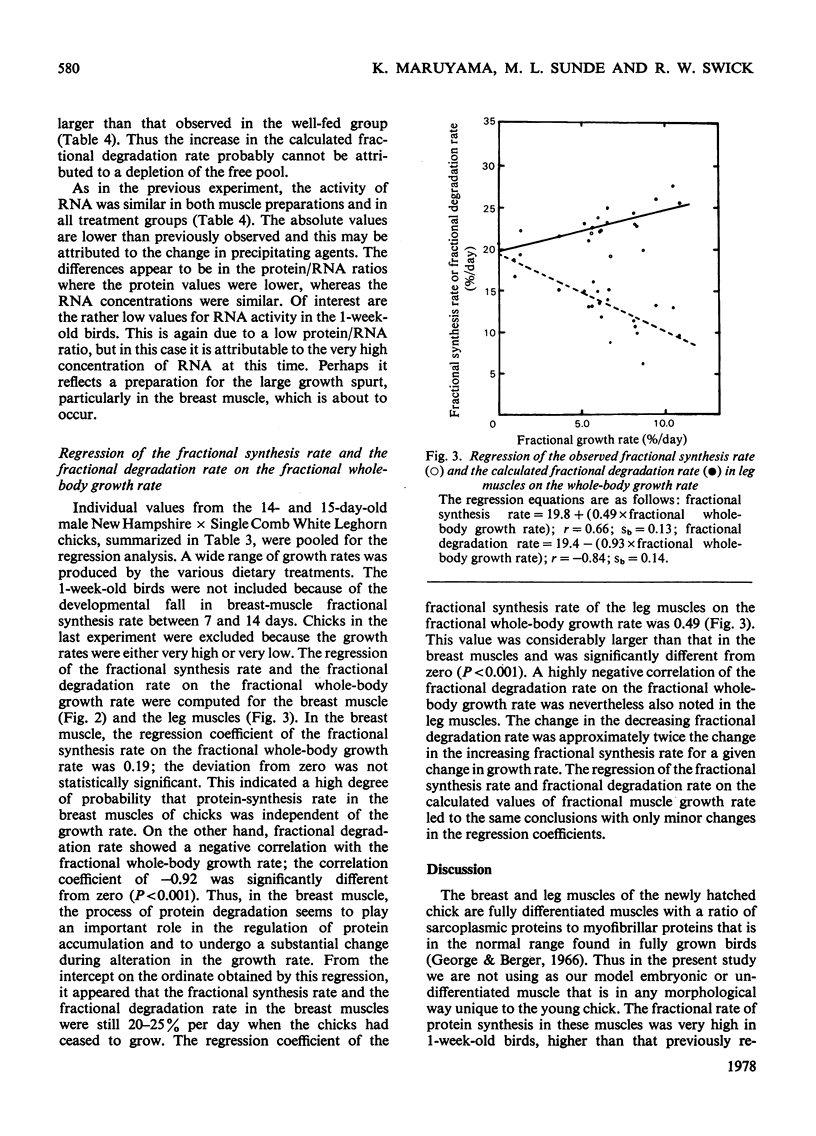
The growth rates of young chicks were varied from 0 to 10% per day by manipulation of the adequacy of the amino acid and energy supply. The rates of protein synthesis in the white breast (pectoralis thoracica) muscle and the dark leg (gastrocnemius and peronaeus longus) muscles were estimated by feeding l-[U-14C]tyrosine in amino acid/agar-gel diets (`dietary infusion'). This treatment rapidly and consistently produced an isotopic equilibrium in the expired CO2 and in the free tyrosine of plasma and the muscles. Wholebody protein synthesis in 2-week-old chicks was estimated from the tyrosine flux and was 6.4g/day per 100g body wt. In 1-week-old chicks the rate of protein synthesis was more rapid in the breast muscles than in the leg muscles, but decreased until the rates were similar in 2-week-old birds. Synthesis was also more rapid in fast-growing Rock Cornish broilers than in medium-slow-growing New Hampshire×Single Comb White Leghorn chicks. No or barely significant decrease in the high rates of protein synthesis, in the protein/RNA ratio and in the activity of RNA for protein synthesis occurred in non- or slow-growing chicks fed on diets deficient in lysine, total nitrogen or energy. Thus the machinery of protein synthesis in the young chick seems to be relatively insensitive to dietary manipulation. In the leg muscles, there was a small but significant correlation between the fractional rate of growth and protein synthesis. A decrease in the fractional rate of degradation, however, appeared to account for much of the accumulation of muscle protein in rapidly growing birds. In addition, the rapid accumulation of breast-muscle protein in rapidly growing chicks appeared to be achieved almost entirely by a marked decrease in the fractional rate of degradation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akinwande A. I., Bragg D. B. Effect of dietary lysine level in a wheat protein diet on growth and changes in DNA, RNA and protein composition in the tissue of broiler chicks. Poult Sci. 1974 Jan;53(1):134–143. doi: 10.3382/ps.0530134. [DOI] [PubMed] [Google Scholar]
- Canolty N. L., Nasset E. S. Intestinal absorption of free and protein-bound dietary methionine in the rat. J Nutr. 1975 Jul;105(7):867–877. doi: 10.1093/jn/105.7.867. [DOI] [PubMed] [Google Scholar]
- Chee P. Y., Swick R. W. Effect of dietary protein and tryptophan and the turnover of rat liver ornithine aminotransferase. J Biol Chem. 1976 Feb 25;251(4):1029–1034. [PubMed] [Google Scholar]
- Garlick P. J., Marshall I. A technique for measuring brain protein synthesis. J Neurochem. 1972 Mar;19(3):577–583. doi: 10.1111/j.1471-4159.1972.tb01375.x. [DOI] [PubMed] [Google Scholar]
- Garlick P. J., Millward D. J., James W. P. The diurnal response of muscle and liver protein synthesis in vivo in meal-fed rats. Biochem J. 1973 Dec;136(4):935–945. doi: 10.1042/bj1360935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlick P. J., Millward D. J., James W. P., Waterlow J. C. The effect of protein deprivation and starvation on the rate of protein synthesis in tissues of the rat. Biochim Biophys Acta. 1975 Nov 18;414(1):71–84. doi: 10.1016/0005-2787(75)90126-4. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L. Protein turnover in skeletal muscle. I. Protein catabolism during work-induced hypertrophy and growth induced with growth hormone. J Biol Chem. 1969 Jun 25;244(12):3217–3222. [PubMed] [Google Scholar]
- Harney M. E., Swick R. W., Benevenga N. J. Estimation of tissue protein synthesis in rats fed diets labeled with (U-14C)tyrosine. Am J Physiol. 1976 Oct;231(4):1018–1023. doi: 10.1152/ajplegacy.1976.231.4.1018. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laurent G. J., Sparrow M. P. Changes in RNA, DNA and protein content and the rates of protein synthesis and degradation during hypertrophy of the anterior latissimus dorsi muscle of the adult fowl (Gallus domesticus). Growth. 1977 Dec;41(4):249–262. [PubMed] [Google Scholar]
- Laurent G. J., Sparrow M. P., Millward D. J. Turnover of muscle protein in the fowl. Changes in rates of protein synthesis and breakdown during hypertrophy of the anterior and posterior latissimus dorsi muscles. Biochem J. 1978 Nov 15;176(2):407–417. doi: 10.1042/bj1760407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K., Harper A. E., Sunde M. L. Effects of D-, DL-and L-glutamic acid on chicks. J Nutr. 1975 Aug;105(8):1012–1019. doi: 10.1093/jn/105.8.1012. [DOI] [PubMed] [Google Scholar]
- Maruyama K., Shands H. L., Harper A. E., Sunde M. L. An evaluation of the nutritive value of new high protein oat varieties (cultivars). J Nutr. 1975 Aug;105(8):1048–1054. doi: 10.1093/jn/105.8.1048. [DOI] [PubMed] [Google Scholar]
- Millward D. J., Garlick P. J., Stewart R. J., Nnanyelugo D. O., Waterlow J. C. Skeletal-muscle growth and protein turnover. Biochem J. 1975 Aug;150(2):235–243. doi: 10.1042/bj1500235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHIMKE R. T. THE IMPORTANCE OF BOTH SYNTHESIS AND DEGRADATION IN THE CONTROL OF ARGINASE LEVELS IN RAT LIVER. J Biol Chem. 1964 Nov;239:3808–3817. [PubMed] [Google Scholar]
- Scornik O. A., Botbol V. Role of changes in protein degradation in the growth of regenerating livers. J Biol Chem. 1976 May 25;251(10):2891–2897. [PubMed] [Google Scholar]
- Scornik O. A. In vivo rate of translation by ribosomes of normal and regenerating liver. J Biol Chem. 1974 Jun 25;249(12):3876–3883. [PubMed] [Google Scholar]
- Shibko S., Koivistoinen P., Tratnyek C. A., Newhall A. R., Friedman L. A method for sequential quantitative separation and determination of protein, RNA, DNA, lipid, and glycogen from a single rat liver homogenate or from a subcellular fraction. Anal Biochem. 1967 Jun;19(3):514–528. doi: 10.1016/0003-2697(67)90242-4. [DOI] [PubMed] [Google Scholar]
- Swick R. W., Ip M. M. Measurement of protein turnover in rat liver with (14C)carbonate. Protein turnover during liver regeneration. J Biol Chem. 1974 Nov 10;249(21):6836–6841. [PubMed] [Google Scholar]
- Turner L. V., Garlick P. J. The effect of unilateral phrenicectomy on the rate of protein synthesis in rat diaphragm in vivo. Biochim Biophys Acta. 1974 Apr 27;349(1):109–113. doi: 10.1016/0005-2787(74)90013-6. [DOI] [PubMed] [Google Scholar]
- WAALKES T. P., UDENFRIEND S. A fluorometric method for the estimation of tyrosine in plasma and tissues. J Lab Clin Med. 1957 Nov;50(5):733–736. [PubMed] [Google Scholar]
- Waterlow J. C., Stephen J. M. The effect of low protein diets on the turn-over rates of serums, liver and muscle proteins in the rat, measured by continuous infusion of L-[14C]lysine. Clin Sci. 1968 Oct;35(2):287–305. [PubMed] [Google Scholar]
- Young V. R., Stothers S. C., Vilaire G. Synthesis and degradation of mixed proteins, and composition changes in skeletal muscle of malnourished and refed rats. J Nutr. 1971 Oct;101(10):1379–1390. doi: 10.1093/jn/101.10.1379. [DOI] [PubMed] [Google Scholar]


