Abstract
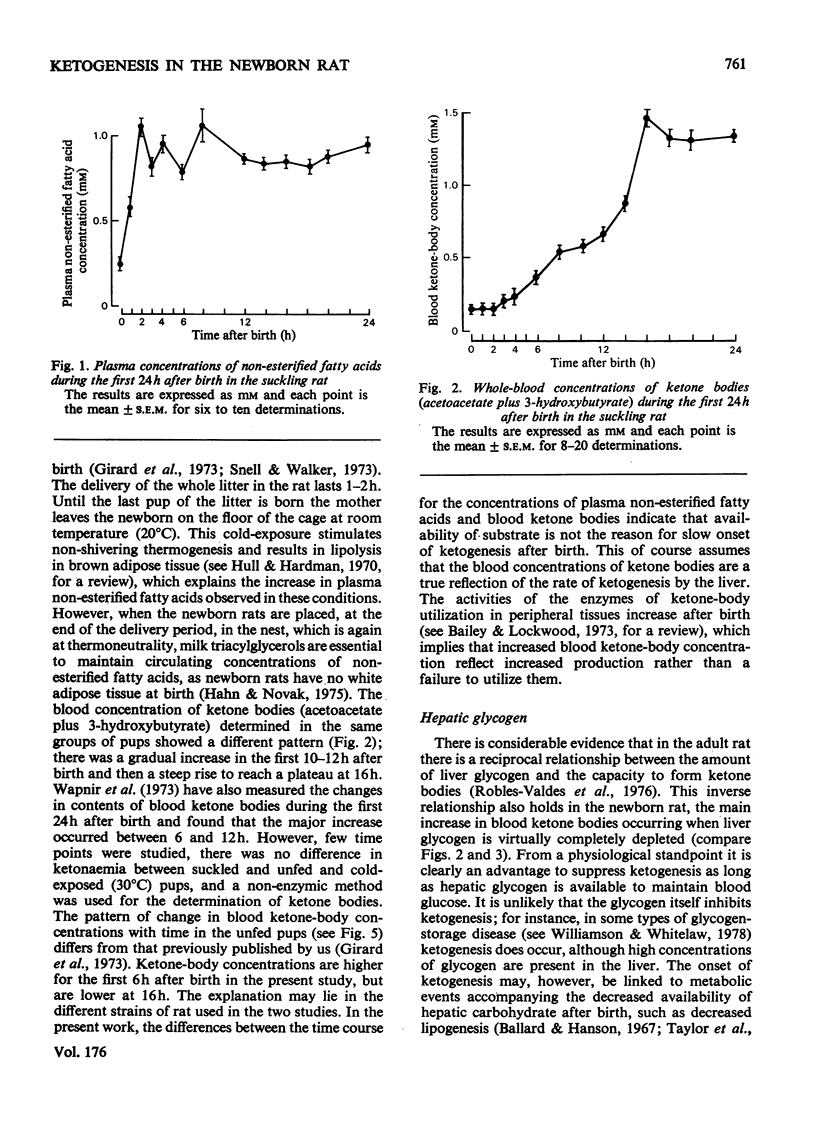
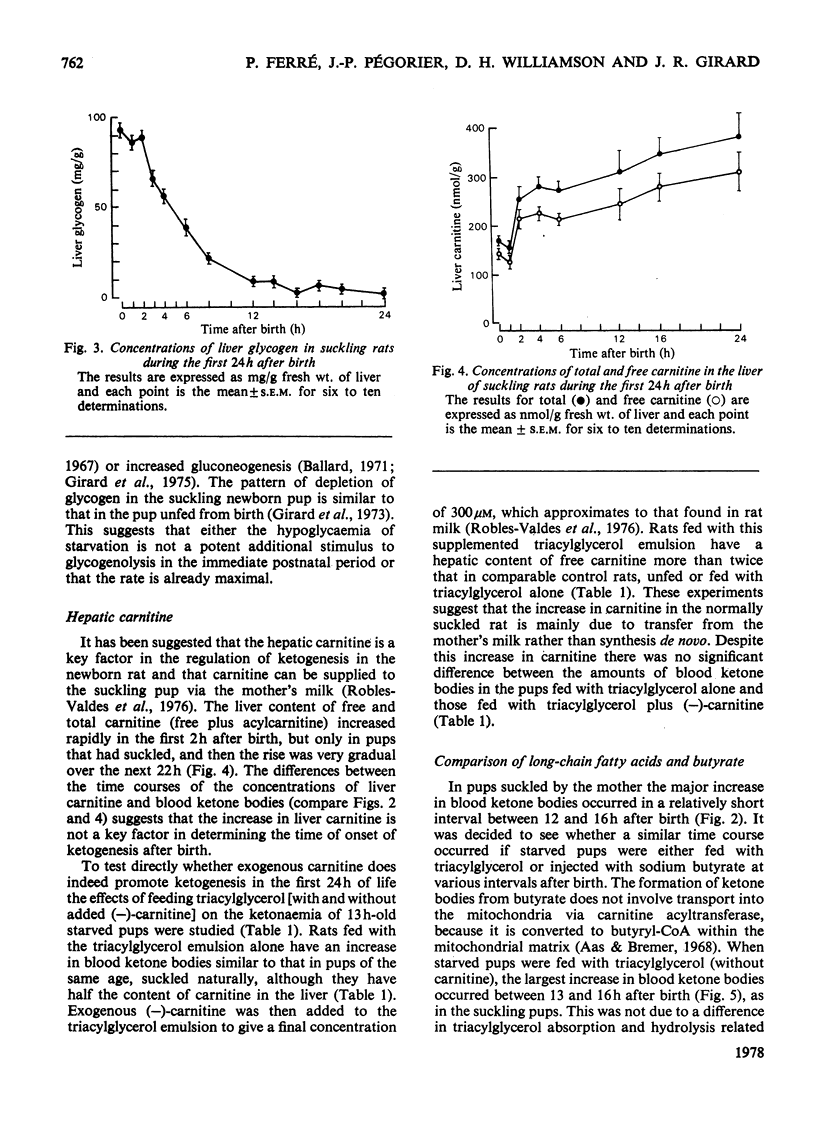
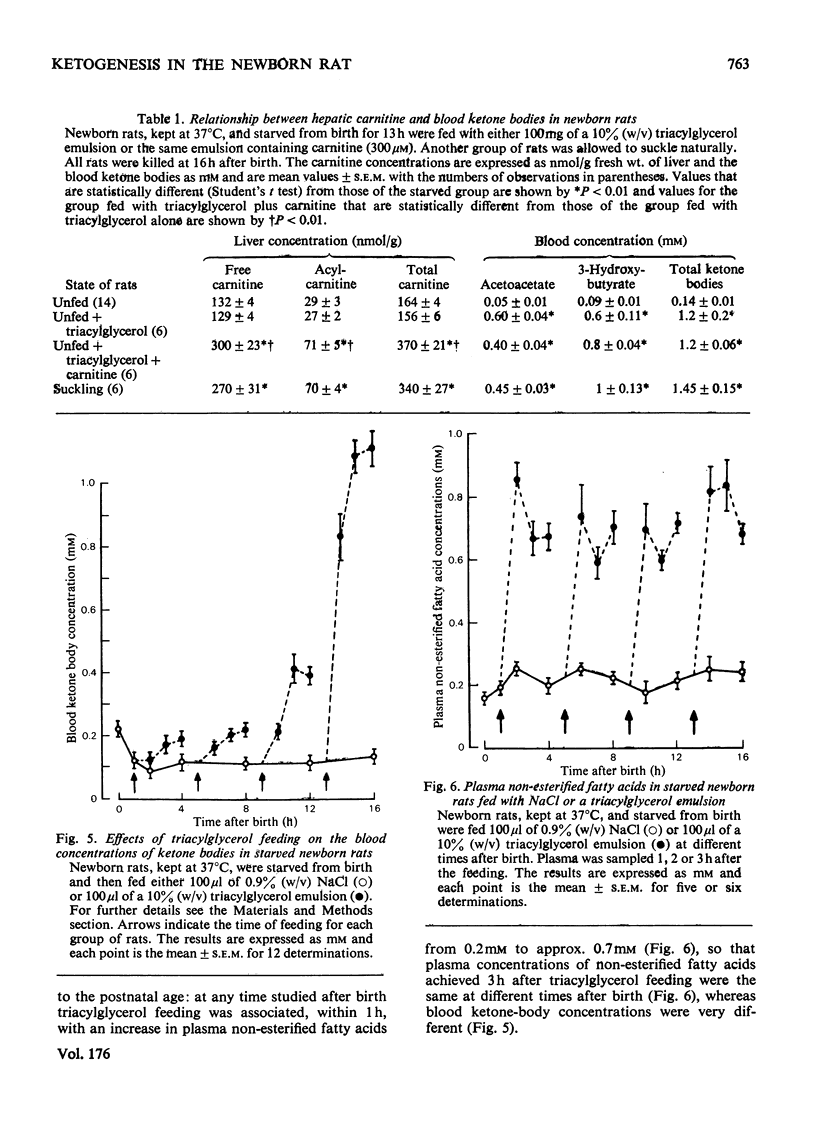
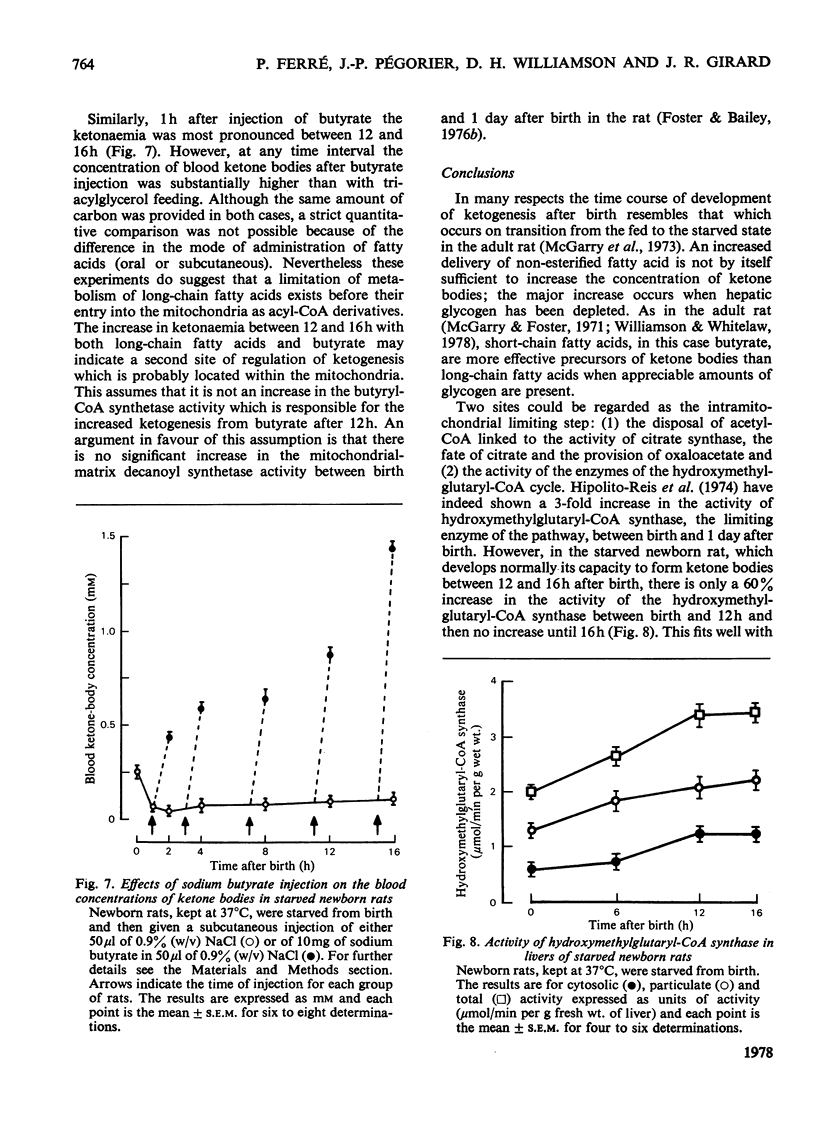
In the suckling newborn rat, blood ketone bodies begin to increase slowly 4h after birth and then rise sharply between 12 and 16h, whereas the major increase in plasma non-esterified fatty acids and liver carnitine occurs during the first 2h of life, parallel with the onset of suckling. In the starved newborn rat, which shows no increase in liver carnitine unless it is fed with a carnitine solution, the developmental pattern of the ketogenic capacity (tested by feeding a triacylglycerol emulsion, which increases plasma non-esterified fatty acids by 3-fold) is the same as in the suckling animal. This suggests that the increases in plasma non-esterified fatty acids and liver carnitine seen 2h after birth in the suckling animal are not the predominant factors inducing the switch-on of ketogenesis. Injection of butyrate to starved newborn pups resulted in a pattern of blood ketone bodies which was similar to that found after administration of triacylglycerols, but, at all time points studied, the hyperketonaemia was more pronounced with butyrate. It is suggested that, even if the entry of long-chain fatty acids into the mitochondria is a rate-limiting step, it is not the only factor controlling ketogenesis after birth in the rat. As in the adult rat, there is a reciprocal correlation between the liver glycogen content and the concentration of ketone bodies in the blood.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aas M., Bremer J. Short-chain fatty acid activation in rat liver. A new assay procedure for the enzymes and studies on their intracellular localization. Biochim Biophys Acta. 1968 Oct 22;164(2):157–166. doi: 10.1016/0005-2760(68)90142-2. [DOI] [PubMed] [Google Scholar]
- Augenfeld J., Fritz I. B. Carnitine palmitolyltransferase activity and fatty acid oxidation by livers from fetal and neonatal rats. Can J Biochem. 1970 Mar;48(3):288–294. doi: 10.1139/o70-050. [DOI] [PubMed] [Google Scholar]
- Bailey E., Lockwood E. A. Some aspects of fatty acid oxidation and ketone body formation and utilization during development of the rat. Enzyme. 1973;15(1):239–253. [PubMed] [Google Scholar]
- Ballard F. J., Hanson R. W. Changes in lipid synthesis in rat liver during development. Biochem J. 1967 Mar;102(3):952–958. doi: 10.1042/bj1020952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drahota Z., Hahn P., Kleinzeller A., Kostolánská A. Acetoacetate formation by liver slices from adult and infant rats. Biochem J. 1964 Oct;93(1):61–65. doi: 10.1042/bj0930061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster P. C., Bailey E. Changes in hepatic fatty acid degradation and blood lipid and ketone body content during development of the rat. Enzyme. 1976;21(5):397–407. doi: 10.1159/000458889. [DOI] [PubMed] [Google Scholar]
- Foster P. C., Bailey E. Changes in the activities of the enzymes of hepatic fatty acid oxidation during development of the rat. Biochem J. 1976 Jan 15;154(1):49–56. doi: 10.1042/bj1540049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard J. R., Cuendet G. S., Marliss E. B., Kervran A., Rieutort M., Assan R. Fuels, hormones, and liver metabolism at term and during the early postnatal period in the rat. J Clin Invest. 1973 Dec;52(12):3190–3200. doi: 10.1172/JCI107519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard J. R., Guillet I., Marty J., Marliss E. B. Plasma amino acid levels and development of hepatic gluconeogenesis in the newborn rat. Am J Physiol. 1975 Aug;229(2):466–473. doi: 10.1152/ajplegacy.1975.229.2.466. [DOI] [PubMed] [Google Scholar]
- Hahn P., Novak M. Development of brown and white adipose tissue. J Lipid Res. 1975 Mar;16(2):79–91. [PubMed] [Google Scholar]
- Ho R. J. Radiochemical assay of long-chain fatty acids using 63Ni as tracer. Anal Biochem. 1970 Jul;36(1):105–113. doi: 10.1016/0003-2697(70)90337-4. [DOI] [PubMed] [Google Scholar]
- KOREN Z., SHAFRIR E. PLACENTAL TRANSFER OF FREE FATTY ACIDS IN THE PREGNANT RAT. Proc Soc Exp Biol Med. 1964 Jun;116:411–414. doi: 10.3181/00379727-116-29263. [DOI] [PubMed] [Google Scholar]
- LUCKEY T. D., MENDE T. J., PLEASANTS J. The physical and chemical characterization of rat's milk. J Nutr. 1954 Nov 10;54(3):345–359. doi: 10.1093/jn/54.3.345. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. Hormonal control of ketogenesis. Biochemical considerations. Arch Intern Med. 1977 Apr;137(4):495–501. [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. The regulation of ketogenesis from octanoic acid. The role of the tricarboxylic acid cycle and fatty acid synthesis. J Biol Chem. 1971 Feb 25;246(4):1149–1159. [PubMed] [Google Scholar]
- McGarry J. D., Mannaerts G. P., Foster D. W. A possible role for malonyl-CoA in the regulation of hepatic fatty acid oxidation and ketogenesis. J Clin Invest. 1977 Jul;60(1):265–270. doi: 10.1172/JCI108764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry J. D., Meier J. M., Foster D. W. The effects of starvation and refeeding on carbohydrate and lipid metabolism in vivo and in the perfused rat liver. The relationship between fatty acid oxidation and esterification in the regulation of ketogenesis. J Biol Chem. 1973 Jan 10;248(1):270–278. [PubMed] [Google Scholar]
- McGarry J. D., Robles-Valdes C., Foster D. W. Role of carnitine in hepatic ketogenesis. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4385–4388. doi: 10.1073/pnas.72.11.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles-Valdes C., McGarry J. D., Foster D. W. Maternal-fetal carnitine relationship and neonatal ketosis in the rat. J Biol Chem. 1976 Oct 10;251(19):6007–6012. [PubMed] [Google Scholar]
- Roehrig K. L., Allred J. B. Direct enzymatic procedure for the determination of liver glycogen. Anal Biochem. 1974 Apr;58(2):414–421. doi: 10.1016/0003-2697(74)90210-3. [DOI] [PubMed] [Google Scholar]
- Shah J., Bailey E. Changes in the activities of the enzymes of hepatic ketogenesis in the rat between late fetal life and weaning. Enzyme. 1977;22(1):35–40. doi: 10.1159/000458505. [DOI] [PubMed] [Google Scholar]
- Snell K., Walker D. G. Glucose metabolism in the newborn rat. Temporal studies in vivo. Biochem J. 1973 Apr;132(4):739–752. doi: 10.1042/bj1320739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. B., Bailey E., Bartley W. Changes in hepatic lipigenesis during development of the rat. Biochem J. 1967 Nov;105(2):717–722. doi: 10.1042/bj1050717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMSON D. H., MELLANBY J., KREBS H. A. Enzymic determination of D(-)-beta-hydroxybutyric acid and acetoacetic acid in blood. Biochem J. 1962 Jan;82:90–96. doi: 10.1042/bj0820090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wapnir R. A., Tildon J. T., Cornblath M. Metabolic differences in offspring of rats fed high-fat and control diets. Am J Physiol. 1973 Mar;224(3):596–599. doi: 10.1152/ajplegacy.1973.224.3.596. [DOI] [PubMed] [Google Scholar]
- Whitelaw E., Williamson D. H. Effects of lactation of ketogenesis from oleate or butyrate in rat hepatocytes. Biochem J. 1977 Jun 15;164(3):521–528. doi: 10.1042/bj1640521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., Bates M. W., Krebs H. A. Activity and intracellular distribution of enzymes of ketone-body metabolism in rat liver. Biochem J. 1968 Jul;108(3):353–361. doi: 10.1042/bj1080353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh Y. Y., Zee P. Insulin, a possible regulator of ketosis in newborn and suckling rats. Pediatr Res. 1976 Mar;10(3):192–197. doi: 10.1203/00006450-197603000-00010. [DOI] [PubMed] [Google Scholar]


